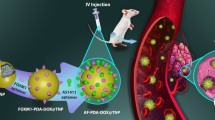
Abstract
Purpose
Development of efficient in vivo delivery nanodevices remains a major challenge to achieve clinical application of siRNA. The present study refers to the conception of core-shell nanoparticles aiming to make possible intravenous administration of chemically unmodified siRNA oriented towards the junction oncogene of the papillary thyroid carcinoma.
Methods
Nanoparticles were prepared by redox radical emulsion polymerization of isobutylcyanoacrylate and isohexylcyanoacrylate with chitosan. The loading of the nanoparticles with siRNA was achieved by adsorption. The biological activity of the siRNA-loaded nanoparticles was assessed on mice bearing a papillary thyroid carcinoma after intratumoral and intravenous administration.
Results
Chitosan-coated nanoparticles with a diameter of 60 nm were obtained by adding 3% pluronic in the preparation medium. siRNA were associated with the nanoparticles by surface adsorption. In vivo, the antisense siRNA associated with the nanoparticles lead to a strong antitumoral activity. The tumor growth was almost stopped after intravenous injection of the antisense siRNA-loaded nanoparticles, while in all control experiments, the tumor size was increased by at least 10 times.
Conclusion
This work showed that poly(alkylcyanoacrylate) nanoparticles coated with chitosan are suitable carriers to achieve in vivo delivery of active siRNA to tumor including after systemic administration.









Similar content being viewed by others
REFERENCES
Kalota A, Shetzline SE, Gewirtz AM. Progress in the development of nucleic acid therapeutics for cancer. Cancer Biol Ther. 2004;3:4–12.
Meyer M, Wagner E. Recent developments in the application of plasmid DNA-based vectors and small interfering RNA therapeutics for cancer. Hum Gene Ther. 2006;17:1062–76.
Russ V, Wagner E. Cell and tissue targeting of nucleic acids for cancer gene therapy. Pharm Res. 2007;24:1047–57.
de Fougerolles AR. Delivery vehicles for small interfering RNA in vivo. Hum Gene Ther. 2008;19:125–32.
Shrivastava N, Srivastava A. RNA interference: an emerging generation of biologicals. Biotechnol J. 2008;3:339–53.
Hutvagner G, Simard MJ. Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol. 2008;9:22–32.
Bertrand JR, Pottier M, Vekris A, Opolon P, Maksimenko A, Malvy C. Comparison of antisense oligonucleotides and siRNA in cell culture and in vivo. Biochem Biophys Res Commun. 2002;296:1000–4.
Downward J. RNA interference. BMJ. 2004;328:1245–8.
Nguyen T, Menocal EM, Harborth J, Fruehauf JH. RNAi therapeutics: an update on delivery. Curr Opin Mol Ther. 2008;10:158–67.
Pirollo KF, Chang EH. Targeted delivery of small interfering RNA: approaching effective cancer therapies. Cancer Res. 2008;68:1247–50.
Fattal E, Barratt G. Nanotechnologies and controlled release systems for the delivery of antisense oligonucleotides and small interfering RNA. Br J Pharmacol. 2009;157:179–94.
de Wolf HK, Snel CJ, Verbaan FJ, Schiffelers RM, Hennink WE, Storm G. Effect of cationic carriers on the pharmacokinetics and tumor localization of nucleic acids after intravenous administration. Int J Pharm. 2007;331:167–75.
Garbuzenko OB, Saad M, Betigeri S, Zhang M, Vetcher AA, Soldatenkov VA, et al. Intratracheal versus intravenous liposomal delivery of siRNA, antisense oligonucleotides and anticancer drug. Pharm Res. 2009;26:382–94.
Am J, Su D, Che O, Li WS, Sun L, Zhang ZY, et al. Functional gene silencing mediated by chitosan/siRNA nanocomplexes. Nanotechnology. 2009;20:405103.
Howard KA. Delivery of RNA interference therapeutics using polycation-based nanoparticles. Adv Drug Deliv Rev. 2009;61:710–20.
Bouclier C, Moine L, Hillaireau H, Marsaud V, Connault E, Opolon P, et al. Physicochemical characteristics and preliminary in vivo biological evaluation of nanocapsules loaded with siRNA targeting estrogen receptor alpha. Biomacromolecules. 2008;9:2881–90.
Bartlett DW, Davis ME. Impact of tumor-specific targeting and dosing schedule on tumor growth inhibition after intravenous administration of siRNA-containing nanoparticles. Biotechnol Bioeng. 2008;99:975–85.
Maksimenko A, Polard V, Villemeur M, Elhamess H, Couvreur P, Bertrand JR, et al. In vivo potentialities of EWS-Fli-1 targeted antisense oligonucleotides-nanospheres complexes. Ann NY Acad Sci. 2005;1058:52–61.
De Martimprey H, Vauthier C, Malvy C, Couvreur P. Polymer nanocarriers for the delivery of small fragments of nucleic acids: oligonucleotides and siRNA. Eur J Pharm Biopharm. 2009;71:490–504.
Wang XL, Xu R, Wu X, Gillespie D, Jensen R, Lu ZR. Targeted systemic delivery of a therapeutic siRNA with a multifunctional carrier controls tumor proliferation in mice. Mol Pharm. 2009;6:738–46.
Toub N, Bertrand JR, Tamaddon A, Elhamess H, Hillaireau H, Maksimenko A, et al. Efficacy of siRNA nanocapsules targeted against the EWS-Fli1 oncogene in Ewing sarcoma. Pharm Res. 2006;23:892–900.
Elhamess H, Bertrand JR, Maccario J, Maksimenko A, Malvy C. Antitumor vectorized oligonucleotides in a model of ewing sarcoma: unexpected role of nanoparticles. Oligonucleotides. 2009;19:255–64.
de Martimprey H, Bertrand JR, Fusco A, Santoro M, Couvreur P, Vauthier C, et al. siRNA nanoformulation against the ret/PTC1 junction oncogene is efficient in an in vivo model of papillary thyroid carcinoma. Nucleic Acids Res. 2008;36(1):e2. doi:10.1093/nar/gkm1094.
Bertholon I, Lesieur S, Labarre D, Besnard M, Vauthier C. Characterization of dextran-poly(isobutylcyanoacrylate) copolymers obtained by redox radical and anionic emulsion polymerization. Macromolecules. 2006;39:3559–67.
Bertholon I, Vauthier C, Labarre D. Complement activation by core-shell poly(isobutylcyanoacrylate)-polysaccharide nanoparticles: influences of surface morphology, length, and type of polysaccharide. Pharm Res. 2006;23:1313–23.
Vonarbourg A, Passirani C, Saulnier P, Benoit JP. Parameters influencing the stealthiness of colloidal drug delivery systems. Biomaterials. 2006;27:4356–73.
Passirani C, Barratt G, Devissaguet JP, Labarre D. Long-circulating nanoparticles bearing heparin or dextran covalently bound to poly(methyl methacrylate). Pharm Res. 1998;15:1046–50.
Decuzzi P, Pasqualini R, Arap W, Ferrari M. Intravascular delivery of particulate systems: does geometry really matter? Pharm Res. 2009;26:235–43.
Bravo-Osuna I, Ponchel G, Vauthier C. Tuning of shell and core characteristics of chitosan-decorated acrylic nanoparticles. Eur J Pharm Sci. 2007;30:143–54.
Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Meth. 1983;65:55–63.
Vauthier C, Schmidt C, Couvreur P. Measurement of the density of polymeric nanoparticulate drug carriers made of poly(alkylcyanoacrylate) and poly(lactic acid) derivatives. J Nanoparticle Res. 1999;1:411–8.
Kim IY, Yoo MK, Kim BC, Kim SK, Lee HC, Cho CS. Preparation of semi-interpenetrating polymer networks composed of chitosan and poloxamer. Int J Biol Macromol. 2006;38:51–8.
Chauvierre C, Leclerc L, Labarre D, Appel M, Marden MC, Couvreur P, et al. Enhancing the tolerance of poly(alkylcyanoacrylate) nanoparticles with surface modular design. Int J Pharm. 2007;338:327–32.
Nemati F, Dubernet C, Colin de Verdière A, Poupon MF, Treupel-Acar L, Puisieux F, et al. Some parameters influencing cytotoxicity of free doxorubicin loaded nanoparticles in sensitive and multidrug resistant leucemic murine cells: incubation time, number of nanoparticles per cell. Int J Pharm. 1994;102:55–62.
Mailänder V, Landfester K. Interaction of nanoparticles with cells. Biomacromolecules. 2009;10:2379–400.
Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11:812–8.
ACKNOWLEDGMENT
We would thank Jeril Degrouard and Danielle Jaillard for the TEM analysis at the CCME Orsay and Bassim Al-Sakere (MD) for his help in animal experimentation. The “Budget Qualité Recherches” of the University of Paris Sud-11 is gratefully acknowledged for the funding of this project as is Henkel Biomedical (Dublin, Ireland) for the gift of isobutylcyanoacrylate and isohexylcyanoacrylate. Henri de Martimprey was supported by a fellowship from the French Ministère de la Recherche et de la Technologie and the French Association pour la Recherche sur le Cancer (ARC).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
de Martimprey, H., Bertrand, JR., Malvy, C. et al. New Core-Shell Nanoparticules for the Intravenous Delivery of siRNA to Experimental Thyroid Papillary Carcinoma. Pharm Res 27, 498–509 (2010). https://doi.org/10.1007/s11095-009-0043-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-0043-8