Abstract
Purpose
A new inhaler (Medspray®) for pulmonary drug delivery based on the principle of Rayleigh break-up has been tested with three different spray nozzles (1.5; 2.0 and 2.5 μm) using aqueous 0.1% (w/w) salbutamol and 0.9% (w/w) sodium chloride solutions.
Materials and methods
Particle size distributions in the aerosol were measured with the principles of time of flight (APS) and laser diffraction (LDA).
Results
The Medspray® inhaler exhibits a highly constant droplet size distribution in the aerosol during dose emission. Droplets on the basis of Rayleigh break-up theory are monodisperse, but due to some coalescence the aerosols from the Medspray® inhaler are slightly polydisperse. Mass median aerodynamic diameters at 60 l.min−1 from APS are 1.42; 1.32 and 1.27 times the theoretical droplet diameters (TD’s) and median laser diffraction diameters are 1.29; 1.14 and 1.05 times TD for 1.5; 2.0 and 2.5 μm nozzles (TD: 2.84; 3.78 and 4.73 μm respectively).
Conclusions
The narrow particle size distribution in the aerosol from the Medspray® is highly reproducible for the range of flow rates from 30 to 60 l.min−1. The mass median aerodynamic droplet diameter can be well controlled within the size range from 4 to 6 μm at 60 l.min−1.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
INTRODUCTION
In the past decades it has become clear that drug deposition targeting to a specific site in the respiratory tract requires sophisticated technology. Irrespective of whether the drug is in the dry or in the wet state, its aerodynamic size distribution, the inspiratory manoeuvre with which the drug is inhaled and the moment of aerosol release in the inhaled air stream are of decisive importance (1). Many different technologies have been presented to produce and deliver dry or wet aerosols under the required conditions. They include various new wet aerosol generation principles (2–4), particle engineering technologies to improve aerosolisation of drug particles in dry powder inhalers (5), techniques that control the inhalation manoeuvre (6) or link the aerosol release to this manoeuvre (7). Several reviews on new principles for wet aerosols have appeared (1–4). They produce so-called ‘soft mist’ aerosols either by mechanical, thermo-mechanical or by electro-mechanical means. The advantages mentioned for such devices are the use of propellant-free (simple) drug solutions and the production of slowly moving aerosols. It has been reported that some of these new nebulisers produce well controlled and rather narrow droplet size distributions (2). However, this depends on the aerosol generation principle used and may be influenced by the inspiratory flow rate and the formulation being nebulised. For example, for the AERx, it has been shown that more than 90% of the particles may be within the narrow size range from 1 to 3 μ (8).
Particles in a narrow size range potentially improve drug targeting in the lungs, providing that the inspiratory flow manoeuvre can be well controlled. It has been shown that peripheral deposition in patients with cystic fibrosis can be maximised by controlling the flow rate between 0.25 and 0.5 l.s−1, using monosized particles of 2–3 μ (6). In a different study with 3 μ particles, it was found that total lung deposition may vary between 20 and 95% when the breathing pattern is not controlled (9). Not only differences in the flow manoeuvre, but also differences in administration devices (10) and patient characteristics (11) may explain why different particle sizes have been recommended for drugs with the same target area, e.g. for salbutamol within the range from 2.8 (12) to 6 μ (13). A particle size in this range for a bronchodilator, for which the target area is in not in the alveolar region but rather in the central and upper airways, has been shown effective in a study with monodisperse aerosols (14). It could be demonstrated that smaller particles (1.5–3 μm) achieve greater total lung deposition, farther distal airway penetration and more peripheral deposition, whereas larger particles (6 μm) result in greater bronchodilatory effect for drugs like salbutamol. However, it was also reported that increasing the flow rate decreases total lung deposition for large particles due to increased oropharyngeal deposition (14).
The aim of this study was to show that the principle of Rayleigh break-up can be a low cost, yet highly effective and robust alternative for near-monodisperse aerosol generation. A simple hand-held, preservative-free, non-pressurised metered dose inhaler (Medspray®) has been developed on the basis of this principle, containing micro engineered nozzles produced with wafer stepper lithography and etching techniques. Different nozzle diameters in different geometries have been tested with two different particle sizing techniques: a laser diffraction apparatus (LDA) and an aerodynamic particle sizer (APS). Also the reproducibility of the delivered dose from the Medspray® was tested.
Medspray® DESIGN AND PRINCIPLE OF OPERATION
The Medspray® inhaler concept consists of a spray nozzle in combination with a specially designed pump system. The liquid is dispersed into droplets by pressing the drug solution through an array of nozzles with mechanical means, and a special mouthpiece that mixes the generated droplets with the air stream inhaled by the patient.
Theoretically, the break-up of a liquid jet from a circular single orifice is in droplets of uniform size if the break-up is within the Rayleigh regime (15). A major contribution to the understanding of jet break-up mechanisms has been given by Weber (16) who extended Rayleigh’s basic analysis to include the viscosity of the liquid. According to Weber, the droplet size for pure water at room temperature is 1.95 times the diameter of the nozzle. The arrangement (number and diameter) of nozzle arrays used for this study is such that the total area for passage of the drug solution is the same for all droplet sizes.
The Medspray® hand-held inhaler is as simple as a metered dose inhaler regarding drug formulation (non-pMDI) and the handling by the patient is similar to that of a pMDI (Fig. 1). This has the advantage that patients being used to administer their medication with an mdi, can directly switch over to the Medspray® without requiring intensive training. One major difference with MDI’s is the relatively low velocity of the aerosol from the Medspray® inhaler however. The aerosol is discharged into a Venturi-like mouthpiece channel inside which mixing of the droplets with the inhaled air occurs. The special shape of the mouthpiece and an air flow resistance on its inlet help to guide the patient towards a flow rate in the range between 30 and 60 l.min−1. The air flow perpendicular to the initial droplet flow prevents impaction of the droplets with the inner mouthpiece walls and also minimizes droplet coalescence. The geometry of the mouthpiece channel slows down the aerosol particles before they leave the mouthpiece and enter the mouth. At 30 l.min−1 the maximum aerosol velocity leaving the mouthpiece is below 4 m.s−1, which is considerably lower than the aerosol velocity from classic (CFC) MDI’s (17). Moreover, particle thrust is entirely determined by the drag force of the inspiratory flow and not by the aerosol generating principle, preventing that the aerosol particles are substantially deposited in the oropharynx.
The Medspray® inhaler is suitable for nebulising any solution with viscosity below 10 mPas, which includes most protein and peptide solutions, and the drug solution is stored in a container with a mechanical pump system. Different nozzles can be used to target a special site in the lung. The patient starts dose administration by inhaling via the mouthpiece. The patient then actuates the inhaler by pressing the dose release button on top of the inhaler which loads the spring between this button and the drug container. The spring load will start the release of the contents of the pump system through the spray nozzle and this makes dose release through the spray nozzle independent of the pressure or speed with which the patient presses the dose release button of the Medspray®. Typically, the dose from the tested Medspray® is 30 μl, which takes about 2 s to be aerosolised. Because of this prolonged release of drug, the hand-lung coordination is not particularly critical. Even if a patient presses the button before the inhalation has been started, most of the generated aerosol will still be delivered to the lungs. At a flow rate of 60 l.min−1, the total dose will be delivered in 2 l of inhaled air which is in agreement with draft guidelines on inhalanda.
MATERIALS AND METHODS
Medspray® inhalers used for the experiments were prototypes prepared for a clinical study. Spray nozzles with three different nozzle diameters were tested with 0.1% (w/w salbutamol) and/or 0.9% (w/w sodium chloride) aqueous solutions. The nozzles were selected on the basis of the outcome of preliminary experiments in which the effect of nozzle array (distance between nozzles and nozzle line-ups) on droplet coalescence was investigated as function of the air velocity through the mouthpiece. The results presented in this study are for spray nozzles of 1.5; 2.0 and 2.5 μm diameter.
Sodium chloride solutions (20 ml ampoules) were purchased (Braun, Germany) and for the salbutamol solutions Ventolin (ampoules with 2.5 mg in 2.5 ml) were purchased (GSK, UK). Viscosities of the solutions were measured with a rotation viscosimeter (Brookfield Engineering Laboratories, van Oortmerssen, The Netherlands) at a rotation speed of 250 rpm. The solutions were used as such or as a mixture with a ratio for salbutamol to sodium chloride of 1:2, yielding a salbutamol dose of 10 μg in 30 μl nebulization liquid. Drug containers with a special pump system were filled in house with 7.5 ml of the prepared drug solutions, which is the quantity necessary for the administration of 250 doses.
Consistency of metered dose from the pump system was measured by weighing the pump before and after each individual emitted dose on an analytical balance with an error percentage of ± 0.033% over the whole range of 200 doses at various flow rates in the range from 20 to 90 l.min−1.
Cumulative volume distributions as function of the droplet diameter for the aerosols from the Medspray® inhaler were measured with a HELOS BF/MAGIC laser diffraction apparatus (Sympatec, Germany) using a special inhaler adapter INHALER 2000 (18). No counter flow nor sheath flow was applied through the adapter. All single measurements were triggered on an optical signal of 0.2% on channel 30 and conducted with a 100 mm (R3) lens for the particle size range 0.9–175 μm. Calculations were based on the Fraunhofer theory because the optical parameters for the drug solutions as function of the droplet size distribution are unknown. It has been shown that Fraunhofer diffraction is quite accurate for predominantly unimodal products and multimodal powders for which the modes are quite close and clearly within the declared limits of the lens (19). Different flow rates were adjusted to study the effect of air velocity through the mouthpiece on droplet evaporation and coalescence. Because the droplets are generated from aqueous solutions with low drug concentrations (density approaches 1 g.cm−3) and because droplets are spherical, the laser diffraction diameters are supposed to match perfectly well the aerodynamic droplet diameters. To follow the droplet size distribution as function of the emission time, time sliced measurements were also conducted with an interval time of 100 ms. Measuring was started manually before a dose was released into the laser beam. Data points for presentation were selected on the basis of the optical concentration to mark the start of dose emission (C opt > 0.25%) and upon time (approx. 2 s) for the end of dose release from the inhaler’s mouthpiece.
For assessing the true aerodynamic diameters of 0.9% sodium chloride droplets (aqueous solution), an aerodynamic particle sizer TSI model 3310 (TSI Inc., USA) was used (with Phantom particle and Stokes correction) in combination with a TSI 100 to 1 aerosol dilutor model 3302A. For these measurements, a special technique was applied. The aerosols from the Medspray® inhalers were sampled in a 10 l cylindrical metal spacer tube with 0.1m inner diameter at flow rates of 30 and 60 l.min−1. While the aerosol was collected, the droplets dried by water evaporation and the dry particles were next introduced to the measuring principle of the APS at a flow rate of 5 l.min−1. As the APS distinguishes individual particles on the basis of time of flight, particle counts (in narrow size classes) are obtained which yield number distributions with a high resolution. This enables to distinguish particles obtained from drying of single droplets, from those derived from multiplets (being the result of droplet coalescence). From the known density (2.2 g.cm−3) and dynamic shape factor (1.08) of the dried cubic sodium chloride particles (20) as well as the original salt concentration in the aerosolised solution (0.9% w/w), the number distributions of dried particles can be re-calculated in the number distributions of the original droplets and ultimately in volume and mass distributions. The mass median aerodynamic diameters thus obtained have been compared with the volume median diameters from laser diffraction measurement.
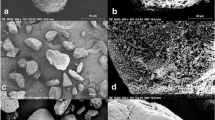
Scanning electron micrographs (SEM pictures) of dried particles for APS-measurement were taken with a Jeol 5610 JP microscope. Particles were sampled by placing the drying tube in vertical position over a collection plate. Aerosol particles entering the tube from the inhaler with controlled air flow dried while travelling through the tube and deposited on the plate. Prior to investigation, the samples were coated with 5 nm of Au/Pd in a sputtering device.
RESULTS AND DISCUSSION
Traditionally, multi-stage cascade impactors and aerodynamic particle sizers are used for medical aerosol characterisation as they yield mass, respectively number distributions as function of the aerodynamic diameter which governs particle deposition in the respiratory tract. It has recently been recognised however, that laser diffraction analysis may produce very meaningful results too (21). This technique has the special advantage of following size distributions in the aerosol continuously with so-called time sliced measurements. Also droplet evaporation and coalescence, both as function of the inspiratory flow rate, can be monitored only with laser diffraction technique. For these reasons, this study was completed using both APS and LDA. Because it was found that the viscosity of the 0.9% saline and 0.1% salbutamol solutions are the same (and equal to that of pure water, being 1.00 mPa.s), the droplet size distributions from the Medspray® nozzles are the same for all three liquids under the same circumstances.
Figure 2 shows an example of the consistency of the dose metered by the pump system for a 0.9% sodium chloride (saline) solution using a nozzle diameter of 2.5 μm. Metered doses are expressed as percent of the targeted value. The variation (maximum and minimum of individual doses) after priming of the nozzle (10 doses) is within the ± 5% range and there is no drop-off in metered dose near the end of lifetime (200 doses). Only within the first ten doses there is a gradual increase towards the targeted value, meaning that the inhaler has to be primed. However, current design improvements are focused on minimising this starting effect. Retention in the mouthpiece of the Medspray® inhaler for all nozzle types in the range of flow rates between 30 and 60 l.min−1 is between 5 and 10% of the metered dose from the nozzle, decreasing the delivered dose from the inhaler to 90–95% of the metered value.
A typical droplet volume distribution curve obtained with the special APS technique (including droplet drying) of an aerosol from a saline solution is shown in Fig. 3. For the example, a 2.5 μm nozzle has been used and the aerosol was drawn into the drying cylinder on the APS with a flow rate of 60 l.min−1. SEM investigation confirmed that the dried particles were perfectly cubic, proving that crystalline solid particles were obtained with known density and dynamic shape factor and that the presented re-calculation into original droplet sizes is correct. On the basis of Rayleigh break-up droplets of approx. 1.9 times the nozzle diameter (2.5 μm) would be expected. Figure 3 shows that the first peak indeed corresponds with a droplet diameter slightly less than 5 μ. A secondary peak is visible at a position just over 6 μm and the peak is skewed on the side of larger particles which is proof for the existence of doublet and triplet droplets in the aerosol as the result of coalescence. By merging of two droplets, the volume is doubled, but the diameter is increased only by a factor 1.26 (d = 5.99 μm). Similarly the diameter of a triplet is only 1.44 times the diameter of a singlet (d = 6.84 μm). This results in different ratios of mass median aerodynamic diameter (MMAD) from APS-measurement to calculated droplet diameter on the basis of Weber’s break-up theory for different nozzles, as shown in Table I. In spite of the occurrence of some coalescence, the droplet size distributions from APS are relatively narrow as presented in Fig. 4, whereas the volume median aerodynamic diameters vary only from 1.23 to 1.38 times the expected theoretical diameters. Because the size distributions are not log-normal, the calculation of geometric standard deviations is arguable. As an alternative, the relative spans of the size distributions have been calculated {(D 90 − D 10) / D 50}, which are presented in Table I too.
Cumulative mass distribution curves as function of the aerodynamic droplet diameter for the aerosols from an aqueous 0.9% sodium chloride solution delivered at an air flow rate of 60 l.min−1 from the Medspray® inhaler into the drying tube of the aerodynamic particle sizer using 1.5; 2.0 and 2.5 μm spray nozzles.
With laser diffraction technique droplets can be measured before they evaporate. For droplets from aqueous drug solutions which almost have unit density and are perfectly spherical, laser diffraction diameters differ not noticeable from aerodynamic diameters. However, size distributions calculated with the Fraunhofer approximation may show inaccuracies for particles in the size range below 1–2 μm, depending on the size distribution in the aerosol (22). Although the Lorenz–Mie model is more complete in terms of describing all possible interactions of the laser beam with the particles, and therefore theoretically more correct, this model can not be applied for drug solutions for which the optical parameters as function of the size distribution are not known.
For the laser diffraction measurements the same inhalers and the same 0.9% w/w sodium chloride solution were used as for the measurements with the APS. A typical volume frequency distribution curve (as function of the diameter) from laser diffraction analysis for the aerosol from the 2.5 μm nozzle spraying an aqueous 0.9% sodium chloride solution at 60 l.min−1 is shown in Fig. 5. The results are not directly comparable with those presented in Fig. 3 from APS showing a number distribution in which particles consisting of an integer number of primary entities produce discrete peaks. The volume frequency distribution curve in Fig. 5 does not show secondary or ternary peaks on the side of the larger particles but some fines in the size range between 1 and 2 micron which are the result of Fraunhofer overestimation of fines. These small particles do not exist as can be concluded from the APS measurement (Fig. 3). For the specific example in Fig. 5, a correction can be made (closed symbols), because the peaks for the imaginary fines and the aerosol particles are completely separated from each other. For the corrected curve, 90% of the total volume is in particles in the size range between 3 and 9 μm and the volume median diameter (VMD) is 5.50 μm. This is quite close to the calculated droplet diameter of single entities (4.88 μm) and only 10% higher than the VMD for the uncorrected curve (4.99 μm for the open symbols). For comparison, the MMAD for the 2.5 μm nozzle from APS measurement is 5.99 μm. For the 1.5 and 2.0 μm nozzles a correction of the laser diffraction data for the imaginary fines is not possible because the peaks for the fines and the aerosol particles partly overlap each other. However, thanks to the much smaller volume median diameters for the aerosols from these nozzles, the occurrence of ‘Fraunhofer fines’ is less pronounced (Fig. 6).
Volume frequency distribution curves as function of the droplet diameter for the aerosol from a 2.5 μm spray nozzle for an aqueous 0.9% sodium chloride solution delivered at an air flow rate of 60 l.min−1 into the adapter of the laser diffraction apparatus from the Medspray® inhaler. Open symbols before, and closed symbols after correction for the Fraunhofer overestimation of fines.
Cumulative volume distribution curves as function of the droplet diameter for the aerosols from an aqueous 0.9% sodium chloride solution delivered at an air flow rate of 60 l.min−1 into the adapter of the laser diffraction apparatus from the Medspray® inhaler using 1.5; 2.0 and 2.5 μm spray nozzles. No corrections made for the Fraunhofer overestimation of fines.
In spite of the calculation of some imaginary fines with the Fraunhofer approximation, the relative spans obtained from laser diffraction technique are even slightly smaller than those obtained from aerodynamic particle sizing (Table II versus Table I). Whereas the data from LDA are of somewhat lower confidence in the size classes for the smallest particles, the APS data presented may show some inaccuracy for the coarsest particles. This could be the result of the applied technique for particle collection and drying in a spacer tube, which may have increased the number of particles being coalesced.
The effect of droplet evaporation on the laser diffraction results has been investigated by elongating the distance between the inhaler’s mouthpiece and the laser beam with a tube of 500 mm. This decreased the median droplet diameter for an aerosol from 0.9% sodium chloride solution at 60 l.min−1 (2.5 μm nozzle) only by 6.2%, whereas the relative span of the size distribution hardly changed (from 1.39 to 1.35). This suggests that no classification (e.g. by sedimentation in the inlet tube) or droplet coalescence occurred during laser diffraction measurement. Also the effects of viscosity and temperature on the droplet size distribution in the aerosol were investigated. It was found that the temperature within the range of values to be expected during normal use has no effect, whereas the viscosity increases the droplet size (< 5% for the drug solutions tested) as predicted by Weber (16). There is an effect of flow rate on droplet coalescence however. This is shown in Fig. 7, comparing the volume median diameters of all three nozzles with each other at 30 and 60 l.min−1 through the Medspray® inhaler. On average for all three nozzles, increasing the flow rate from 30 to 60 l.min−1 decreases the volume median droplet diameter by 11.4%, corresponding with a reduction of droplet mass by 38%. This is the result of a considerable reduction in the number of multiplets, which may partly compensate for the increased losses in the oropharynx when the flow rate is increased from 30 to 60 l.min−1. That the decrease in volume median diameter is not the result of droplet evaporation seems to be confirmed by the observation that particularly the X90-value is reduced.
Effect of the air flow rate on the volume median droplet diameter for aerosols from the Medspray® inhaler using 1.5; 2.0 and 2.5 μm nozzles for spraying a 0.9% aqueous sodium chloride solution. Data are the mean of two measurements with laser diffraction technique. No corrections made for the Fraunhofer overestimation of fines.
The results from time sliced (laser diffraction) measurement show that the droplet size distribution is extremely constant during dose emission. For the example of the 2.5 μm nozzle spraying a 30 μl salbutamol solution at a nebulization rate of 15 μl.s−1 (air flow rate through the Medspray® inhaler is 30 l.min−1), this is shown in Fig. 8. The figure also shows the high reproducibility in droplet size distribution between two duplicate experiments.
Time sliced measurements yielding the median droplet size (circles) and optical concentration in the aerosol (diamonds) as function of the emission time (2 s) for the Medspray® inhaler with a 2.5 μm nozzle spraying a salbutamol: saline solution in a ratio 1: 2. Most error bars for the volume median diameter, indicating the spread between two duplicate experiments at 30 l.min−1, are within the size of the symbols.
CONCLUSIONS
The results from this study show that the principle of Rayleigh break-up can be used to aerosolise drug solutions for inhalation very effectively. A simple, low cost, hand-held inhaler (Medspray®) has been made on the basis of this principle, which has the simplicity (in handling) of a metered dose inhaler. The inhaler exhibits excellent consistency of delivered dose and the spring loaded dose release guarantees that delivery rate (for the current design: 30 μl in 2 s) and particle size distribution in the aerosol are very constant. The two different techniques used to characterize the aerosol from the Medspray® inhaler, both produced polydisperse particle size distributions in a narrow size range, although particles on the basis of the Weber break-up theory are expected to be of uniform size. To find out whether this is the result of errors introduced by the measuring techniques used for the study, or primarily that of droplet coalescence, the effect of flow rate on droplet coalescence was investigated. It was found that increasing the flow rate through the mouthpiece decreases the degree of coalescence as it changes the distance between the droplets in the flow direction. The degree of coalescence is also influenced by the nozzle array and the droplet inertia. This is the reason why the ratio of MMAD (or VMD) to theoretical diameter was found to increase with decreasing droplet diameter. Also a correction for the imaginary fines obtained with Fraunhofer approximation for LDA as sizing technique was made. For the 2.5 μm nozzle, it was found that the correction does not change the polydisperse distribution into monodisperse particles, although from this correction the relative span of the aerosol from the 2.5 μm nozzle is reduced from 1.4 to 0.8. In terms of geometric standard deviation (GSD), which has not been presented for reasons explained before (and is given only as indicative now), the corresponding value would be less than 1.4 (for spherical particles GSD has unity). The results show that within the range of flow rates from 30 to 60 l.min−1 the droplet diameter from the Medspray® can be well controlled and is in fair agreement with the theoretical droplet diameter, in spite of the coalescence. This makes the Medspray® a robust and highly reproducible hand-held inhaler of which the aerosol properties can easily be changed to meet the requirements for targeting of a specific site of deposition in the human respiratory lungs, simply by exchanging the spray nozzle.
Abbreviations
- APS:
-
Aerodynamic particle sizer
- GSD:
-
Geometric standard deviation
- LDA:
-
Laser diffraction analysis (apparatus)
- MDI:
-
Metered dose inhaler
- MMAD:
-
Mass median aerodynamic diameter
- TD:
-
Theoretical diameter on the basis of Rayleigh break-up
- VMD:
-
Volume median diameter (also X50 from laser diffraction analysis)
References
P. R. Byron. Drug delivery devices. Proc. Am. Thorax Soc. 1:321–328 (2004).
O. Nerbrink. http://www.touchbriefings.com/pdf/1859/nurbrink.pdf (accessed 30/03/07) Novel liquid delivery systems—what is out there today. Future Drug Delivery (2006).
M. Dolovich. New propellant-free technologies under investigation. J. Aerosol Med. 12:S9–S17 (1999).
W. H. Frijlink and A. H. de Boer. Trends in the technology-driven development of new inhalation devices. Drug Discov. Today 2:47–57 (2005).
S. A. Shoyele and S. Cawthorne. Particle engineering techniques for inhaled biopharmaceuticals. Adv. Drug Del. Reviews 58:1009–1029 (2006).
P. Brand, T. Meyer, S. Häussermann, M. Schulte, G. Scheuch, T. Bernhard, B. Sommerauer, N. Weber, and M. Griese. Optimum peripheral drug deposition in patients with cystic fibrosis. J. Aerosol Med. 18:45–54 (2005).
R. E. Van Dyke, and K. Nikander. Delivery of iloprost inhalation solution with the HaloLite, Prodose and I-neb adaptive aerosol delivery systems: an in vitro study. Respir. Care 52:184–190 (2007).
I. Gonda, J. A. Schuster, R. M. Rubsamen, P. Lloyd, D. Cipolla, and S. J. Farr. Inhalation delivery systems with compliance and disease management capabilities. J. Controlled Release 53:269–274 (1998).
P. Brand, I. Friemel, T. Meyer, H. Schulz, J. Heyder, and K. Häußinger. Total deposition of therapeutic particles during spontaneous and controlled inhalations. J. Pharm. Sci. 89:724–731 (2000).
W. DeHaan and W. H. Finlay. In vitro monodisperse aerosol deposition in a mouth and throat with six different inhalation devices. J. Aerosol Med. 14:361–367 (2001).
W. D. Bennett and K. L. Zeman. Effect of body size on breathing pattern and fine-particle deposition in children. J. Appl. Physiol. 97:821–826 (2004).
P. Zanen, L. T. Go, and J-W. Lammers. The optimal particle size for β-adrenergic aerosols in mild asthmatics. Int. J. Pharm. 107:211–217 (1994).
O. Usmani, M. F. Biddiscombe, J. A. Nightingale, S. R. Underwood, and P. J. Barnes. Effects of bronchodilator particle size in asthmatic patients using monodisperse aerosols. J. Appl. Physiol. 95:2106–2112 (2003).
O. S. Usmani, M. F. Biddiscombe, and P. J. Barnes. Regional lung deposition and bronchodilator response as a function of β2-agonist particle size. Am. J. Respir. Crit. Care Med. 172:1497–1504 (2005).
Lord Rayleigh (J.W. Strutt). On the instability of jets. Proc. London Math. Soc. 10:4–13 (1878).
C. Weber. Disintegration of liquid jets. Z. Angew. Math. Mech. 11:136–159 (1931).
D. Hochrainer, H. Hölz, C. Kreher, L. Scaffidi, M. Spallek, and H. Wachtel. Comparison of the aerosol velocity and spray duration of Respimat® Soft Mist™ inhaler and pressurized metered dose inhalers. J. Aerosol Med. 18:273–282 (2005).
A. H. de Boer, D. Gjaltema, P. Hagedoorn, M. Schaller, W. Witt, and H. W. Frijlink. Design and application of a new modular adapter for laser diffraction characterization of inhalation aerosols. Int. J. Pharm. 249:233–245 (2002).
A. Annapragada and A. Adjei. An analysis of the Fraunhofer diffraction method for particle size distribution analysis and its application to aerosolized sprays. Int. J. Pharm. 127:219–227 (1996).
W. C. Hinds. Aerosol Technology. Properties, behaviour, and measurement of airborne particles, John Wiley & Sons, New York, 1982, pp. 38–68.
J. P. Mitchell, M. W. Nagel, S. Nichols, and O. Nerbrink. Laser diffractometry as a technique for the rapid assessment of aerosol particle size from inhalers. J. Aerosol Med. 19:409–430 (2006).
R. H. Müller and R. Schuhmann. Teilchengröbenmessung in der Laborpraxis, Wissenschaftliche Verlagsgesellschaft, Stuttgart, 1996, pp. 55–75.
Acknowledgments
The authors wish to thank Aero Pump GmbH (Hochheim, Germany) for their materials supply and technical support. They are also obliged to the Dutch Ministry of Economic Affairs (The Hague) for their financial support of the projects (Microdruppels and TSGE2057), to the provinces of Overijssel and Gelderland for their support of the ‘Microdruppels’ project and to Marco Schwegler of SenterNovem for his unrestricted support to the project.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
de Boer, A.H., Wissink, J., Hagedoorn, P. et al. In Vitro Performance Testing of the Novel Medspray® Wet Aerosol Inhaler Based on the Principle of Rayleigh Break-up. Pharm Res 25, 1186–1192 (2008). https://doi.org/10.1007/s11095-007-9503-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-007-9503-1