Purpose
The purpose of the study was to investigate the relationship of the second virial coefficient, B 22, to the extent of irreversible protein aggregation upon storage.
Methods
A monoclonal antibody and ovalbumin were incubated at 37°C (3 months) under various solution conditions to monitor the extent of aggregation. The B 22 values of these proteins were determined under similar solution conditions by a modified method of flow-mode static light scattering. The conformation of these proteins was studied using circular dichroism (CD) spectroscopy and second-derivative Fourier transform infrared spectroscopy.
Results
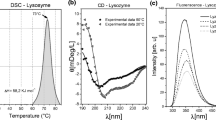
Both proteins readily aggregated at pH 4.0 (no aggregation observed at pH 7.4); the extent of aggregation varied with the ionic strength and the presence of cosolutes (sucrose, glycine, and Tween 80). Debye plots of the monoclonal antibody showed moderate attractive interactions at pH 7.4, whereas, at pH 4.0, nonlinear plots were obtained, indicating self-association. CD studies showed partially unfolded structure of antibody at pH 4.0 compared with that at pH 7.4. In the case of ovalbumin, similar B 22 values were obtained in all solution conditions irrespective of whether the protein aggregated or not. CD studies of ovalbumin indicated the presence of a fraction of completely unfolded as well as partially unfolded species at pH 4.0 compared with that at pH 7.4.
Conclusions
The formation of a structurally altered state is a must for irreversible aggregation to proceed. Because this aggregation-prone species could be an unfolded species present in a small fraction compared with that of the native state or it could be a partially unfolded state whose net interactions are not significantly different compared with those of the native state, yet the structural changes are sufficient to lead to long-term aggregation, it is unlikely that B 22 will correlate with long-term aggregation.








Similar content being viewed by others
References
J. L. Cleland M. F. Powell S. J. Shire (1993) ArticleTitleThe development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation Crit. Rev. Ther. Drug Carr. Syst. 10 307–377 Occurrence Handle1:CAS:528:DyaK2cXhsVektro%3D
J. Brange (2000) Physical stability of proteins S. Frokhaer L. Hovgaard (Eds) Pharmaceutical Formulation Development of Peptides and Proteins Taylor & Francis London 89–112
H. Schellekens (2005) ArticleTitleFactors influencing the immunogenicity of therapeutic proteins Nephrol. Dial. Transplant. 20 3–9
C. J. Roberts (2003) ArticleTitleKinetics of irreversible protein aggregation: analysis of extended Lumry-Eyring models and implications for predicting protein shelf life J. Phys. Chem. B. 107 1194–1207 Occurrence Handle1:CAS:528:DC%2BD3sXivF2qtw%3D%3D Occurrence Handle10.1021/jp026827s
M. Sukumar S. M. Storms M. R. Felippis ParticleDe (2005) ArticleTitleNon-native intermediate conformational states of human growth hormone in the presence of organic solvents Pharm. Res. 22 789–796 Occurrence Handle15906175 Occurrence Handle1:CAS:528:DC%2BD2MXkt1Omsr8%3D Occurrence Handle10.1007/s11095-005-2596-5
R. Wetzel (1994) ArticleTitleMutations and off-pathway aggregation of proteins Trends Biotechnol. 12 193–198 Occurrence Handle7764903 Occurrence Handle1:CAS:528:DyaK2MXhvVOgsA%3D%3D Occurrence Handle10.1016/0167-7799(94)90082-5
S. Krishnan E. Y. Chi J. N. Webb B. S. Chang D. Shan M. Goldenberg M. C. Manning T. W. Randolph J. F. Carpenter (2002) ArticleTitleAggregation of granulocyte colony stimulating factor under physiological conditions: characterization and thermodynamic inhibition Biochemistry 41 6422–6431 Occurrence Handle12009905 Occurrence Handle1:CAS:528:DC%2BD38XjtFahtrc%3D Occurrence Handle10.1021/bi012006m
B. S. Kendrick J. F. Carpenter J. L. Cleland T. W. Randolph (1998) ArticleTitleA transient expansion of the native state precedes aggregation of recombinant human interferon-γ Proc. Natl. Acad. Sci. USA 95 14142–14146 Occurrence Handle9826667 Occurrence Handle1:CAS:528:DyaK1cXns1Gmtbk%3D Occurrence Handle10.1073/pnas.95.24.14142
R. Lumry H. Eyring (1954) ArticleTitleConformation changes of proteins J. Phys. Chem. 58 110–120 Occurrence Handle1:CAS:528:DyaG2cXjsVOmug%3D%3D Occurrence Handle10.1021/j150512a005
S. W. Raso J. Abel J. M. Barnes K. M. Maloney G. Pipes M. J. Treuheit J. King D. N. Brems (2005) ArticleTitleAggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state Protein Sci. 14 2246–2257 Occurrence Handle16131655 Occurrence Handle1:CAS:528:DC%2BD2MXpvVKlsrw%3D Occurrence Handle10.1110/ps.051489405
J. L. Cleland D. I. C. Wang (1993) ArticleTitleCosolvent effects on refolding and aggregation A.C.S. Symp. Ser. 516 151–166 Occurrence Handle1:CAS:528:DyaK3sXht1Sgurk%3D Occurrence Handle10.1021/bk-1993-0516.ch012
A. Kjellsson I. Sethson B.-H. Jonsson (2003) ArticleTitleHydrogen exchange in a large 29 kD protein and characterization of molten globule aggregation by NMR Biochemistry 42 363–374 Occurrence Handle12525163 Occurrence Handle1:CAS:528:DC%2BD38XpsF2gt7g%3D Occurrence Handle10.1021/bi026364g
K. Boren H. Grankvist P. Hammarstrom U. Carlsson (2004) ArticleTitleReshaping the folding energy landscape by chloride salt: impact on molten-globule formation and aggregation behavior of carbonic anhydrase FEBS Lett. 566 95–99 Occurrence Handle15147875 Occurrence Handle1:CAS:528:DC%2BD2cXktVOltbk%3D Occurrence Handle10.1016/j.febslet.2004.03.105
L. A. Kueltzo C. R. Middaugh (2003) ArticleTitleStructural characterization of bovine granulocyte colony stimulating factor: effect of temperature and pH J. Pharm. Sci. 92 1793–1804 Occurrence Handle12949998 Occurrence Handle1:CAS:528:DC%2BD3sXntFymsbY%3D Occurrence Handle10.1002/jps.10440
V. K. Sharma D. S. Kalonia (2003) ArticleTitleTemperature- and pH-induced multiple partially unfolded states of recombinant human interferon-α2a: possible implications in protein stability Pharm. Res. 20 1721–1729 Occurrence Handle14661914 Occurrence Handle1:CAS:528:DC%2BD3sXptVChtrk%3D Occurrence Handle10.1023/B:PHAM.0000003367.62900.0f
D. Bulone V. Martorana P. L. Biagio ParticleSan (2001) ArticleTitleEffects of intermediates on aggregation of native bovine serum albumin Biophys. Chem. 91 61–69 Occurrence Handle11403884 Occurrence Handle1:CAS:528:DC%2BD3MXkt1yis78%3D Occurrence Handle10.1016/S0301-4622(01)00155-7
M. Karlsson L.-G. Martensson P. Olofsson U. Carlsson (2004) ArticleTitleCircumnavigating misfolding traps in the energy landscape through protein engineering: suppression of molten globule and aggregation in carbonic anhydrase Biochemistry 43 6803–6807 Occurrence Handle15157114 Occurrence Handle1:CAS:528:DC%2BD2cXjsVShs7w%3D Occurrence Handle10.1021/bi049709z
V. N. Uversky J. Li A. L. Fink (2001) ArticleTitleEvidence for a partially folded intermediate in alpha-synuclein fibril formation J. Biol. Chem. 276 10737–10744 Occurrence Handle11152691 Occurrence Handle1:CAS:528:DC%2BD3MXjvFCqtLs%3D Occurrence Handle10.1074/jbc.M010907200
S. Yoshioka Y. Aso K.-I. Izutsu S. Kojima (1994) ArticleTitleIs stability prediction possible for protein drugs? Denaturation kinetics of β-galactosidase in solution Pharm. Res. 11 1721–1725 Occurrence Handle7899234 Occurrence Handle1:CAS:528:DyaK2MXis1Cgtrk%3D Occurrence Handle10.1023/A:1018955031042
C. J. Roberts R. T. Darrington M. B. Whitley (2003) ArticleTitleIrreversible aggregation of recombinant bovine granulocyte-colony stimulating factor (BG-CSF) and implications for predicting protein shelf life J. Pharm. Sci. 92 1095–1111 Occurrence Handle12712430 Occurrence Handle1:CAS:528:DC%2BD3sXjs1ehs7c%3D Occurrence Handle10.1002/jps.10377
L. R. Young ParticleDe A. L. Fink K. A. Dill (1993) ArticleTitleAggregation of globular proteins Acc. Chem. Res. 26 614–620 Occurrence Handle10.1021/ar00036a002
W. Liu T. Cellmer D. Keerl J. M. Prausnitz H. W. Blanch (2005) ArticleTitleInteractions of lysozyme in guanidinium chloride solutions from static and dynamic light-scattering measurements Biotechnol. Bioeng. 90 482–490 Occurrence Handle15778988 Occurrence Handle1:CAS:528:DC%2BD2MXktFegsro%3D Occurrence Handle10.1002/bit.20442
R. N. Keener and R. A. Hart. Impact of conformational and colloidal stability on ultrafiltration–diafiltration. Abstracts of Papers, 229th ACS National Meeting, San Diego, CA, USA, March 13–17, 2005. BIOT-271 (2005).
J. Zhang X. Y. Liu (2003) ArticleTitleEffect of protein–protein interactions on protein aggregation kinetics J. Chem. Phys. 119 10972–10976 Occurrence Handle1:CAS:528:DC%2BD3sXovVSns7g%3D Occurrence Handle10.1063/1.1622380
E. Y. Chi S. Krishnan B. S. Kendrick B. S. Chang J. F. Carpenter T. W. Randolph (2003) ArticleTitleRoles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony-stimulating factor Protein Sci. 12 903–913 Occurrence Handle12717013 Occurrence Handle1:CAS:528:DC%2BD3sXjtl2jt78%3D Occurrence Handle10.1110/ps.0235703
J. G. S. Ho A. P. J. Middelberg P. Ramage H. P. Kocher (2003) ArticleTitleThe likelihood of aggregation during protein renaturation can be assessed using the second virial coefficient Protein Sci. 12 708–716 Occurrence Handle12649429 Occurrence Handle1:CAS:528:DC%2BD3sXisVeksbg%3D Occurrence Handle10.1110/ps.0233703
J. G. S. Ho and A. P. J. Middelberg. Aggregation during refolding is dependent on the second virial coefficient. Abstracts of Papers, 225th ACS National Meeting, New Orleans, LA, United States, March 23–27, 2003. BIOT-035 (2003).
J. G. S. Ho A. P. J. Middelberg P. Ramage H. P. Kocher (2003) ArticleTitleThe likelihood of aggregation during protein renaturation can be assessed using the second virial coefficient Protein Sci. 12 708–716 Occurrence Handle12649429 Occurrence Handle1:CAS:528:DC%2BD3sXisVeksbg%3D Occurrence Handle10.1110/ps.0233703
S.-L. Huang F.-Y. Lin C.-P. Yang (2005) ArticleTitleMicrocalorimetric studies of the effects on the interactions of human recombinant interferon-α2a Eur. J. Pharm. Sci. 24 545–552 Occurrence Handle15784344 Occurrence Handle1:CAS:528:DC%2BD2MXisFGktrg%3D Occurrence Handle10.1016/j.ejps.2005.01.003
B. H. Zimm (1946) ArticleTitleApplication of the methods of molecular distribution to solutions of large molecules J. Chem. Phys. 14 164–179 Occurrence Handle1:CAS:528:DyaH28XhsleitQ%3D%3D Occurrence Handle10.1063/1.1724116
W. G. McMillan SuffixJr. J. E. Mayer (1945) ArticleTitleThe statistical thermodynamics of multicomponent systems J. Chem. Phys. 13 276–305 Occurrence Handle1:CAS:528:DyaH2MXisFSktw%3D%3D Occurrence Handle10.1063/1.1724036
B. L Neal D. Asthagiri A. M. Lenhoff (1998) ArticleTitleMolecular origins of osmotic second virial coefficients of proteins Biophys. J. 75 2469–2477 Occurrence Handle9788942 Occurrence Handle1:CAS:528:DyaK1cXntFKktrk%3D
T. L. Hill (1953) ArticleTitleAdsorption on proteins and interactions between protein molecules in solution J. Chem. Phys. 21 1395–1396 Occurrence Handle1:CAS:528:DyaG3sXntVeqsQ%3D%3D Occurrence Handle10.1063/1.1699230
G. Scatchard A. Gee J. Weeks (1954) ArticleTitlePhysical chemistry of protein solutions. Vi. The osmotic pressures of mixtures of human serum albumin and γ-globulins in aqueous sodium chloride J. Phys. Chem. 58 783–787 Occurrence Handle1:CAS:528:DyaG2MXltVKg Occurrence Handle10.1021/j150519a019
S. Lapanje C. Tanford (1967) ArticleTitleProteins as random coils. IV. Osmotic pressures, second virial coefficients, and unperturbed dimensions in 6 M guanidine hydrochloride J. Am. Chem. Soc. 89 5030–5033 Occurrence Handle6074806 Occurrence Handle1:CAS:528:DyaF2sXkvFKru7Y%3D Occurrence Handle10.1021/ja00995a035
A. George Y. Chiang B. Guo A. Arabshahi Z. Cai W. W. Wilson (1997) ArticleTitleSecond virial coefficient as predictor in protein crystal growth Methods Enzymol. 276 100–110 Occurrence Handle1:CAS:528:DyaK2sXivFehs7Y%3D Occurrence Handle10.1016/S0076-6879(97)76052-X
C. Haas J. Drenth W. W. Wilson (1999) ArticleTitleRelation between the solubility of proteins in aqueous solutions and the second virial coefficient of the solution J. Phys. Chem. B 103 2808–2811 Occurrence Handle1:CAS:528:DyaK1MXhvFSjsr8%3D Occurrence Handle10.1021/jp984035l
B. Guo S. Kao H. McDonald A. Asanov L. L. Combs W. W. Wilson (1999) ArticleTitleCorrelation of second virial coefficients and solubilities useful in protein crystal growth J. Cryst. Growth 196 424–433 Occurrence Handle1:CAS:528:DyaK1MXjtVGktQ%3D%3D Occurrence Handle10.1016/S0022-0248(98)00842-2
E. Chi Y. S. Krishnan B. S. Kendrick B. S. Chang J. F. Carpenter T. W. Randolph (2003) ArticleTitleRoles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony-stimulating factor Protein Sci. 12 903–913 Occurrence Handle12717013 Occurrence Handle1:CAS:528:DC%2BD3sXjtl2jt78%3D Occurrence Handle10.1110/ps.0235703
H. Bajaj V. K. Sharma D. S. Kalonia (2004) ArticleTitleDetermination of second virial coefficient of proteins using a dual-detector cell for simultaneous measurement of scattered light intensity and concentration in SEC-HPLC Biophys. J. 87 4048–4055 Occurrence Handle15465853 Occurrence Handle1:CAS:528:DC%2BD2cXhtVOmtbbK Occurrence Handle10.1529/biophysj.104.048686
N. Sreerama. CDPro: A Software Package for Analyzing Protein Spectra. http://lamar.colostate.edu/~sreeram/CDpro/index.html.
J. D. Andya, C. C. Hsu, and S. J. Shire. Mechanisms of aggregate formation and carbohydrate excipient stabilization of lyophilized humanized monoclonal antibody formulations. AAPS PharmSci 5 (2003).
A. Dong S. J. Prestrelski S. D. Allison J. F. Carpenter (1995) ArticleTitleInfrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation J. Pharm. Sci. 84 415–424 Occurrence Handle7629730 Occurrence Handle1:CAS:528:DyaK2MXksVygt74%3D
P. Doty M. Gellert B. Rabinovitch (1952) ArticleTitleThe association of insulin. I. Preliminary investigations J. Am. Chem. Soc. 74 2065–2069 Occurrence Handle1:CAS:528:DyaG38XkvVOqsw%3D%3D Occurrence Handle10.1021/ja01128a063
R. Townend S. N. Timasheff (1960) ArticleTitleMolecular interactions in β-lactoglobulin. III. Light-scattering investigation of the stoichiometry of the association between pH 3.7 and 5.2 J. Am. Chem. Soc. 82 3168–3174 Occurrence Handle1:CAS:528:DyaF3MXlvFyi Occurrence Handle10.1021/ja01497a046
A. W. P. Vermeer W. Norde (2000) ArticleTitleThe thermal stability of immunoglobulin: unfolding and aggregation of a multi-domain protein Biophys. J. 78 394–404 Occurrence Handle10620303 Occurrence Handle1:CAS:528:DC%2BD3cXktFeqsQ%3D%3D Occurrence Handle10.1016/S0006-3495(00)76602-1
O. B. Ptitsyn (1995) ArticleTitleMolten globule and protein folding Adv. Protein Chem. 47 83–229 Occurrence Handle8561052 Occurrence Handle1:CAS:528:DyaK28XlvFOgsg%3D%3D Occurrence Handle10.1016/S0065-3233(08)60546-X
A. L. Fink (1995) ArticleTitleCompact intermediate states in protein folding Annu. Rev. Biophys. Biomol. Struct. 24 495–522 Occurrence Handle7663125 Occurrence Handle1:CAS:528:DyaK2MXmt1amsb8%3D Occurrence Handle10.1146/annurev.bb.24.060195.002431
D. N. Brems S. M. Plaisted E. W. Kauffman H. A. Havel (1986) ArticleTitleCharacterization of an associated equilibrium folding intermediate of bovine growth hormone Biochemistry 25 6539–6543 Occurrence Handle3790541 Occurrence Handle1:CAS:528:DyaL28Xls1Gitbg%3D Occurrence Handle10.1021/bi00369a030
C. Tanford (1961) Thermodynamics C. Tanford (Eds) Physical Chemistry of Macromolecules Wiley New York 202–204
Acknowledgments
The authors gratefully acknowledge financial support and donation of the monoclonal antibody from Pfizer Biologics (St. Louis, MO, USA) and financial support from the National Science Foundation Industry/University Cooperative Research Center for Pharmaceutical Processing (http://www.ipph.purdue.edu/~nsf/aboutCPPR.html).
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Model for Self-Association and Data Analysis of Nonlinear Debye Plots
A monomer–dimer equilibrium is written as
where the association constant K dim is defined as
where [c d] is the molar concentration of the dimer and [c m] is the molar concentration of the monomer. The total molar concentration [c t] of the protein can be written in terms of the monomer concentration as
Combining Eqs. (13) and (14), solving the resulting quadratic equation for positive solution of [c m] and [c d], and converting molar concentration to grams per milliliter, the monomer and dimer concentrations can be written as
For an associating system, the Debye equation is written as
where M av is the weight average molecular weight of all the species present in the solution. Note that B 22 has been substituted with the term B to represent the nonideality arising from monomer–monomer, monomer–dimer, and dimer–dimer interactions. For an associating system, the change in the chemical potential of the solvent with solute concentration is written as (50)
Substituting for c m and c d from Eqs. (16) and (17), taking partial derivatives, and using the result in the derivation of the Rayleigh's light scattering equation, the following Debye equation is obtained:
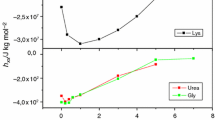
Equation (20) was used to fit the curved Debye plots for the parameters K dim, M m, and B.
Rights and permissions
About this article
Cite this article
Bajaj, H., Sharma, V.K., Badkar, A. et al. Protein Structural Conformation and Not Second Virial Coefficient Relates to Long-Term Irreversible Aggregation of a Monoclonal Antibody and Ovalbumin in Solution. Pharm Res 23, 1382–1394 (2006). https://doi.org/10.1007/s11095-006-0018-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-006-0018-y