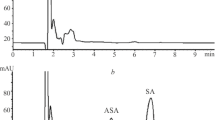
A method for anilocaine determination in rat blood plasma using HPLC with UV-detection was developed. Samples were prepared by liquid—liquid extraction with 1-BuOH—hexane (2:98, v/v) followed by stripping with aqueous formic acid (0.1%). Chromatographic analysis used a Nucleodur HILIC column eluted in isocratic mode by a mobile phase of MeCN and aqueous ammonium formate (25 mM) (85:15) and UV detection at 205 nm. The method was validated for selectivity, carry-over, calibration curve linearity, precision, accuracy, limit of quantitation, dilution integrity, and stability. The analytical range was 25 – 1,000 ng/mL. The proposed method could be applied to pharmacokinetic studies of anilocaine preparations.





Similar content being viewed by others
References
N. A. Gornova, S. V. Chashchina, and V. I. Pantsurkin, in: Abstracts of Papers of an Interdepartmental Scientific-Practical Conference [in Russian], Perm (2000), pp. 39 – 40.
M. S. Gaisinovich, E. E. Stolyarov, et al., Probl. Ekspert. Med., No. 3, 25 – 27 (2004).
V. A. Ryzhikova, A. A. Tikhobaeva, L. A. Salomatina, et al., Perspekt. Mater., No. 2, 26 – 32 (2014).
A. A. Pospelova, T. L. Malkova, and L. N. Karpova, Biomed. Zh., 13, 94 – 102 (2012).
E. E. Stolyarov, Yu. N. Karpenko, and T. L. Malkova, Sud.-Med. Ekspert., No. 3, 24 – 27 (2009).
A. N. Mironov (ed.), Handbook for Preclinical Drug Trials [in Russian], Part 1, Grif i K, Moscow (2012), p. 844.
Guidance for Industry. Bioanalytical Method Validation, Washington (2013).
Guideline on Bioanalytical Method Validation, London (2011).
Author information
Authors and Affiliations
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 52, No. 2, pp. 54 – 58, February, 2018.
Rights and permissions
About this article
Cite this article
Ryzhikova, V.A., Kursakov, S.V., Belov, V.Y. et al. Development and Validation of an HPLC-UV Method for Anilocaine Determination in Blood Plasma. Pharm Chem J 52, 166–170 (2018). https://doi.org/10.1007/s11094-018-1784-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-018-1784-7