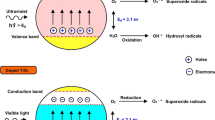
Abstract
In this work, the process of decomposition of 2,4-dichlorophenol (2,4-DCP) vapor under the influence of atmospheric pressure DBD in oxygen was studied. The studies were carried out in two modes: with a catalyst (natural vermiculite doped with zirconium) and without it. A number of basic characteristics of the catalyst were assessed. The rates and effective rate constants of sorption processes, as well as decomposition processes in plasma and plasma-catalytic systems, were determined. Based on these data, the energy efficiency of the decomposition process was calculated. The data obtained suggested that the initial stage of decomposition is the reaction of interaction of electrons with pollutant molecules. The catalyst has been shown to speed up the decomposition process, increase energy efficiency and the conversion of 2,4-DCP to CO2 molecules, and prevent the formation of condensed products on the reactor walls. The work estimates the carbon and chlorine balances before and after treatment, which reach a maximum of 99 and 60%, respectively. It was also shown that the catalyst retains its activity for at least 7 h of continuous operation.






Similar content being viewed by others
Availability of Data and Material
All data generated or analyzed during this study are included in this published article in results section.
References
Huang B, Lei C, Wei C, Zeng G (2014) Chlorinated volatile organic compounds (Cl-VOCs) in environment-sources, potential human health impacts, and current remediation technologies. Environ Int 71:118–138. https://doi.org/10.1016/j.envint.2014.06.013
Li C, Zhao Y, Song H, Li H (2020) A review on recent advances in catalytic combustion of chlorinated volatile organic compounds. J Chem Technol Biotechnol 95:2069–2082. https://doi.org/10.1002/jctb.6308
Lyu X, Guo H, Wang Y, Zhang F, Nie K, Dang J, Liang Z, Dong S, Zeren Y, Zhou B (2020) Hazardous volatile organic compounds in ambient air of China. Chemosphere 246:125731. https://doi.org/10.1016/j.chemosphere.2019.125731
Helmig D, Bottenheim J, Galbally IE, Lewis A, Milton MJT, Penkett S, Plass-Duelmer C, Reimann S, Tans P, Thiel S (2009) Volatile organic compounds in the global atmosphere. Eos 90:513–514. https://doi.org/10.1029/2009EO520001
Fan M-Y, Zhang Y-L, Lin Y-C, Li L, Xie F, Hu J, Mozaffar A, Cao F (2021) Source apportionments of atmospheric volatile organic compounds in Nanjing, China during high ozone pollution season. Chemosphere 263:128025. https://doi.org/10.1016/j.chemosphere.2020.128025
Hanif NM, Hawari NSSL, Othman M, Abd Hamid HH, Ahamad F, Uning R, Ooi MCG, Wahab MIA, Sahani M, Latif MT (2021) Ambient volatile organic compounds in tropical environments: potential sources, composition and impacts—A review. Chemosphere 285:131355. https://doi.org/10.1016/j.chemosphere.2021.131355
Ahlborg UG, Thunberg TM (1980) Chlorinated phenols: occurrence, toxicity, metabolism, and environmental impact. A systematic review of intermediates and their characterization methods in VOCs degradation by different catalytic technologies. Crit Rev Toxicol 7:1–35. https://doi.org/10.3109/10408448009017934
Zhao Z, Ma S, Gao B, Bi F, Qiao R, Yang Y, Wu M, Zhang X (2023) A systematic review of intermediates and their characterization methods in VOCs degradation by different catalytic technologies. Sep Purif Technol 314:123510. https://doi.org/10.1016/j.seppur.2023.123510
Guo Y, Wen M, Li G, An T (2021) Recent advances in VOC elimination by catalytic oxidation technology onto various nanoparticles catalysts: a critical review. Appl Catal 281:119447. https://doi.org/10.1016/j.apcatb.2020.119447
Stanmore BR (2004) The formation of dioxins in combustion systems. Combust Flame 136:398–427. https://doi.org/10.1016/j.combustflame.2003.11.004
Buekens A, Huang H (1998) Comparative evaluation of techniques for controlling the formation and emission of chlorinated dioxins/furans in municipal waste incineration. J Hazard Mater 62:1–33. https://doi.org/10.1016/S0304-3894(98)00153-8
Chen HL, Lee HM, Chen SH, Chang MB, Yu SJ, Li SN (2009) Removal of volatile organic compounds by single-stage and two-stage plasma catalysis systems: a review of the performance enhancement mechanisms, current status, and suitable applications. Environ Sci Technol 43:2216–2227. https://doi.org/10.1021/es802679b
Sultana S, Vandenbroucke AM, Leys C, De Geyter N, Morent R (2015) Abatement of VOCs with alternate adsorption and plasma-assisted regeneration: a review. Catalysts 5:718–746. https://doi.org/10.3390/catal5020718
Thevenet F, Sivachandiran L, Guaitella O, Barakat C, Rousseau A (2014) Plasma–catalyst coupling for volatile organic compound removal and indoor air treatment: a review. J Phys D Appl Phys 47:224011. https://doi.org/10.1088/0022-3727/47/22/224011
Whitehead JC (2010) Plasma catalysis: a solution for environmental problems. Pure Appl Chem 82:1329–1336. https://doi.org/10.1351/PAC-CON-10-02-39
Kim H-H, Teramoto Y, Ogata A, Takagi H, Nanba T (2016) Plasma catalysis for environmental treatment and energy applications. Plasma Chem Plasma Process 36:45–72. https://doi.org/10.1007/s11090-015-9652-7
Gushchin AA, Grinevich VI, Kozlov AA, Kvitkova EY, Shutov DA, Rybkin VV (2017) Destruction of 2,4 dichlorophenol in an atmospheric pressure dielectric barrier discharge in oxygen. Plasma Chem Plasma Process 37:1331–1341. https://doi.org/10.1007/s11090-017-9828-4
Bodrikov IV, Titov EY, Serov AI, Titov DY, Kurskii YA, Ivashkin EG (2023) Low-voltage electron-induced reaction of chlorobenzene in liquid phase. High Energy Chem 57:515–521. https://doi.org/10.1134/S0018143916060023
Fiorenza R (2020) Bimetallic catalysts for volatile organic compound oxidation. Catalysts 10:661. https://doi.org/10.3390/catal10060661
Kamal MS, Razzak SA, Hossain MM (2016) Catalytic oxidation of volatile organic compounds (VOCs)-A review. Atmos Environ 140:117–134. https://doi.org/10.1016/j.atmosenv.2016.05.031Getrightsandcontent
Huang H, Xu Y, Feng Q, Leung DY (2015) Low temperature catalytic oxidation of volatile organic compounds: a review. Catal Sci Technol 5:2649–2669. https://doi.org/10.1039/C4CY01733A
He C, Cheng J, Zhang X, Douthwaite M, Pattisson S, Hao Z (2019) Recent advances in the catalytic oxidation of volatile organic compounds: a review based on pollutant sorts and sources. Chem Rev 119:4471–4568. https://doi.org/10.1021/acs.chemrev.8b00408
Liu R, Wu H, Shi J, Xu X, Zhao D, Ng YH, Zhang M, Liu S, Ding H (2022) Recent progress on catalysts for catalytic oxidation of volatile organic compounds: a review. Catal Sci Technol 12:6945–6991. https://doi.org/10.1039/d2cy01181f
Liotta LF (2010) Catalytic oxidation of volatile organic compounds on supported noble metals. Appl Catal 100:403–412. https://doi.org/10.1016/j.apcatb.2010.08.023
Mu Y, Williams PT (2022) Recent advances in the abatement of volatile organic compounds (VOCs) and chlorinated-VOCs by non-thermal plasma technology: a review. Chemosphere 308:136481. https://doi.org/10.1016/j.chemosphere.2022.136481
Zhao Q, Liu Q, Zheng Y, Han R, Song C, Ji N, Ma D (2020) Enhanced catalytic performance for volatile organic compound oxidation over in-situ growth of MnOx on Co3O4 nanowire. Chemosphere 244:125532
National Environmental Research Center (1974) Methods for chemical analysis of water and wastes. US environmental protection agency, office of technology transfer, ohio
Gushchin AA, Grinevich VI, Shulyk VY, Kvitkova EY, Rybkin VV (2018) Destruction kinetics of 2,4 dichlorophenol aqueous solutions in an atmospheric pressure dielectric barrier discharge in oxygen. Plasma Chem Plasma Process 38:123–134. https://doi.org/10.1007/s11090-017-9857-z
Gordina NE, Melnikov AA, Gusev GI, Gushchin AA, Rumyantsev RN, Astrakhantseva IA (2022) Mechanochemical and plasmachemical processing in the synthesis of catalytic systems based on vermiculite and zirconium oxychloride. ChemChemTech 65:43–57. https://doi.org/10.6060/ivkkt.20226505.6612
Lawton SA, Phelps AV (1978) Excitation of the b1Σg+ state of O2 by low energy electrons. J Chem Phys 69:1055–1068
da Costa RF, de Oliveira EM, Bettega MHF, Varella MTdN, Jones DB, Brunger MJ, Blanco F, Colmenares R, Limão-Vieira P, García G (2015) Electron collisions with phenol: total, integral, differential, and momentum transfer cross sections and the role of multichannel coupling effects on the elastic channel. J Chem Phys 142:104304. https://doi.org/10.1063/1.4913824
Gushchin AA, Grinevich VI, Izvekova TV, Kvitkova EY, Tyukanova KA, Rybkin VV (2021) Decomposition of carbon tetrachloride under the action of a dielectric barrier discharge of atmospheric pressure in an oxygen atmosphere. Chemosphere 270:129392. https://doi.org/10.1016/j.chemosphere.2020.129392
Smirnov SA, Shutov DA, Bobkova ES, Rybkin VV (2015) Physical parameters and chemical composition of a nitrogen DC discharge with water cathode. Plasma Chem Plasma Process 35:639–657. https://doi.org/10.1007/s11090-015-9626-9
Bobkova E, Khodor Y, Kornilova O, Rybkin V (2014) Chemical composition of plasma of dielectric barrier dischargeat atmospheric pressure with a liquid electrode. High Temp 52:511–517. https://doi.org/10.1134/S0018151X14030055
Acknowledgements
The work was supported by the Ministry of Higher Education and Science of the Russian Federation, project no. FZZW-2023-0010 using facilities of the Center for Shared Use of Scientific Equipment at the Ivanovo State University of Chemistry and Technology (with the support of the Ministry of Higher Education and Science of Russia, agreement no. 075-15-2021-671).
Funding
The work was supported by the Ministry of Higher Education and Science of the Russian Federation, project no. FZZW-2023-0010.
Author information
Authors and Affiliations
Contributions
All authors contributed to the writing of this study. Data collection and analysis were performed by K.A. Lapshova, G.I. Gusev and E.Yu. Kvitkova. A.A. Gushchin, V.V. Rybkin and V.V. Grinevich wrote background and discussion parts, made calculations and drawing figures and tables. T.V. Izvekova and N.E. Gordina were responsible for the designing and literature search. The first draft of the manuscript was written by V.V. Grinevich. A.A. Gushchin along with the other authors reviewed and edited all versions of the manuscript. A.A. Gushchin was also responsible for project administration and supervision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethical Approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for Publication
We authors accept and confirm responsibility for releasing this material on behalf of any and all co-authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lapshova, K.A., Gordina, N.E., Kvitkova, E.Y. et al. Destruction of 2,4-Dichlorophenol Vapor in a Process Involving the Combined Action of DBD in Oxygen and a Catalyst. Plasma Chem Plasma Process 44, 853–865 (2024). https://doi.org/10.1007/s11090-024-10462-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-024-10462-y