Abstract
Gas–liquid interfacial plasmas (GLIPs), specifically atmospheric-pressure plasmas (APPs) interacting with liquids, have garnered global interest for potential applications across various fields where reactive oxygen and nitrogen species (RONS) in both the gas and liquid phases could play a key role. However, APP-induced gas- and liquid-phase chemical reactions display spatially nonuniform features and involve a number of species; thus, they are extremely complicated and have not been fully understood and controlled. Herein, our primary focus is centered on elucidating RONS transport processes in GLIPs without direct plasma-liquid contact to reduce the complexity of this mechanism. Firstly, this review delineates the simplified transport models commonly found in general GLIP systems, including: (1) the transport of remotely generated gas-phase RONS to the liquid phase; (2) liquid-phase diffusion governing dissolution into the liquid phase and volatilization loss to the gas phase; and (3) chemical reactions in the liquid phase governing the generation and loss of short-lived RONS. Second, we delve into RONS transport using our laboratory-built plasma devices, aimed at sterilizing plant pathogens, interpreting results in line with the relevant transport models to aid the comprehension of the heterogeneous transport of RONS. Third, we discussed the innovative control of the plasma reaction process in the gas phase required to selectively synthesize N2O5, which is highly reactive at the gas–liquid interface. Finally, future prospects for the efficient utilization of unique reactions at the plasma/gas–liquid interface are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atmospheric pressure plasmas (APPs) directly or remotely interacting with liquids, hereinafter referred to as gas–liquid interfacial plasmas (GLIPs), are widely used in medical, agricultural, and environmental applications [1,2,3,4,5,6,7,8,9,10]. GLIPs utilize the electrical, chemical, mechanical, and thermal effects in the discharge plasma, rendering them a versatile tool for a range of applications. Reactive oxygen and nitrogen species (RONS), particularly those in the liquid phase, are particularly important in GLIP applications involving biological targets, such as microorganisms, cells, and tissues [6, 7, 11, 12]. RONS in these applications are generated and transported into the liquid phase, serving as a source of stimuli for the biological targets [1, 2, 13].
The distinctive capability to produce short-lived RONS at concentration levels significantly impactful on biology, solely from air and water using electricity, distinguishes it from other RONS generation techniques like radiolysis and electrolysis. The generation of short-lived RONS in the liquid phase has been emphasized by many groups [8, 14,15,16,17,18,19,20,21,22] and in our previous studies within the context of sterilization [23, 24], virus inactivation [25, 26], cell membrane permeabilization (gene/molecular transfer to cells) [27,28,29,30,31,32,33], and activation of plant immunity [34, 35]. It should be highlighted that a few exhaustive analyses of plasma-treated water based on chemical kinetics identified the generation of short-lived peroxynitrous acid/peroxynitrite (HOONO/ONOO−) and peroxynitric acid/peroxynitrate (HOONO2/O2NOO−) in a uniform media, revealing their remarkable antibacterial effects in the liquid phase for the first time [18, 19, 36].
Short-lived RONS in the liquid phase are generated concurrently with the complicated transport process of RONS across the gas–liquid interface in GLIPs [2]. The transport of a significant amount of RONS across the gas–liquid interface in GLIPs can result in a spatially nonuniform chemical reaction field, which includes the production of short-lived RONS in the liquid phase (RONSaq) [37, 38]. In addition to non-uniformity, liquid surface motion and morphology may constitute complex feedback mechanisms between the plasma and liquid, called plasma–liquid interactions [2]. The complexity of the nonuniform chemistry and RONS transport across the phases in the plasma gas–liquid interaction presents a challenge in providing a comprehensive and descriptive representation of short-lived RONSaq generation in GLIP, despite their noble nature and crucial roles in applications.
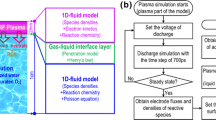
The reduction of complexity in experiments enables the construction of a simplified fundamental model for RONS transport in the GLIP, which can be a building block for constructing a comprehensive and descriptive model for short-lived RONS generation in the GLIP. Therefore, our primary focus has been on GLIP systems that do not involve direct plasma–liquid contact, as they share common underlying mechanisms of RONS transport across the phases in GLIP, which include the following:
-
1.
Transport of remotely generated gaseous RONS (RONSgas) to the liquid phase.
-
2.
Diffusion in the liquid phase that may govern the bidirectional transport of RONS via dissolution into the liquid phase and volatilization loss to the gas phase.
-
3.
Chemical reactions in the liquid phase for generation and loss of RONSaq.
Each model built for the above individual RONS transport mechanisms offers a degree of quantitative insight into the generation and transport of short-lived RONSaq, which was used in our plasma agricultural application studies. It should be emphasized that air APPs with water (air–water GLIPs) can specifically meet agricultural applications, where large-scale treatment at a low cost is frequently required. The precise transport control of RONS derived exclusively from air and water is crucial to meet the demand for agricultural applications by efficiently utilizing short-lived RONS at the target.
This review first presents a model of RONS transport across the gas–liquid interface in “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” section, which serves as a valuable tool for understanding and controlling the behavior of RONSaq. In “Application Systems of Gas–Liquid Interface Transport of Air Plasma-Generated RONS for Plant Disease Control” section, specific examples of RONS transport using our laboratory-built air plasma devices are presented for plant pathogen control [39], which is a plasma agricultural application. The discussion is provided with interpretations from the RONS transport models presented in “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” section and extended to a GLIP in direct contact with air plasma and water in “Air Plasma-Irradiated Solution (APIS) Spraying System” section. These practices ultimately enhance the understanding of RONS transport across phases and lead to the discovery of an interfacial reaction involving dinitrogen pentoxide (N2O5) in the dissolution process into the liquid phase. This dissolution of N2O5 is of considerable significance in agricultural applications because its reaction residue is chemically inert nitrate (NO3−aq), a nitrogen fertilizer. Finally, to ultimately develop a comprehensive and descriptive representation of short-lived RONSaq generation in GLIP, challenges in the experimental validation of short-lived RONS at the gas–liquid interface and their efficient utilization are discussed as future prospects in “Future Prospects on Gas–Liquid Interfacial Air Plasmas” section.
Simplified Modeling of RONS Transport Across Gas–Liquid Interface
Our consistent intention for nearly 10 years has been to temporarily activate RONSaq effects through effective utilization of the air–water GLIP [23,24,25, 34, 35, 40, 41]. In “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” section, simplified transport models of RONSgas into pure water without plasma–liquid contact are presented and formulated. As depicted in Fig. 1, various RONSgas are simultaneously transported to the gas–liquid interface. The isolation of the plasma from the liquid surface inevitably suppresses the transport of neutral and charged species originating in the plasma contact with water, resulting in the formation of a reactive gas containing multiple RONSgas, called plasma effluent gas. Chemical reactions in the plasma effluent gas subsequently merge these species into a stable RONSgas composition, perhaps in pseudo-reaction equilibrium.
In this review, RONSgas in contact with the liquid surface is intentionally reduced to O3gas, H2O2gas, HO2gas, OHgas, NOgas, NO2gas, HNO2gas, N2O5gas, and HNO3gas, as these have been previously established in our previous studies to be crucial in the transport of RONS derived from air and water [23, 24, 40,41,42,43]. The significance of other RONSgas must be examined for each GLIP system and should not be overlooked.
Even with the above simplification, the transport process of RONS across the gas–liquid interface involves a complex network of reactions among a number of species that are interconnected in both space and time, making it highly complicated. Ultimately aiming to establish a descriptive model of RONS transport across the phases, in this review, three RONS transport phenomena are highlighted and individually described in “Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface” to “Reaction Loss in the Liquid Phase” sections, as schematically shown in Fig. 2. “Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface” section describes the mass balance equations for RONS transport processes across the gas–liquid interface. “Diffusion Loss of RONSaq to the Gas Phase” and “Reaction Loss in the Liquid Phase” sections highlight the diffusion and reaction processes of the RONSaq, respectively. These processes play a significant role in the loss of RONSaq in the liquid phase and are concurrently involved in RONSaq generation. This enabled us to delve into an in-depth discussion of the temporally and spatially evolving RONS transport process across the gas–liquid interface, as discussed in “Application Systems of Gas–Liquid Interface Transport of Air Plasma-Generated RONS for Plant Disease Control” section.
Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface
RONSgas, transported to the liquid phase, dissolves into the liquid phase across the gas–liquid interface layer and then initiates inhomogeneous liquid-phase chemistry. Although this non-uniformity is a key characteristic in plasma chemistry, it brings extra complexity and hinders reaction analysis by assuming a uniform reaction process, as in radical chemistry [44, 45]. In this section, a series of formulations that are adaptable to the inhomogeneous reaction chemistry induced by molecular transport across the gas–liquid interface are presented.
-
Molecular transport phenomena across phases
For a clear discussion of RONS transport, we must start with the continuity equation for species \(i\) in both the gas and liquid phases, as follows:
where the molecular flux \({{\varvec{\Gamma}}}_{\varvec{i}}={n}_{i}{{\varvec{u}}}_{{\varvec{i}}}-{D}_{i}\nabla {n}_{i}\) includes convection with the velocity \({{\varvec{u}}}_{{\varvec{i}}}\) and diffusion terms. \({\dot{\omega }}_{i}\) denotes the reaction term for species \(i\). \({D}_{i}\) is the diffusion coefficient of the species \(i\), as listed in Table 1.
In this section, the transport of RONS into a liquid with a finite volume of \({V}_{liq}\) is discussed; thus, Eq. (1) was integrated over the liquid volume \({V}_{liq}\). The integrated continuity equation provides the mass balance of the liquid volume \({V}_{liq}\), which is of practical importance, particularly in experiments involving inhomogeneous reaction chemistry.
where \({S}_{liq}\) denotes the liquid surface of \({V}_{liq}\). The surface normal vector \({\varvec{n}}\) provides the direction from the liquid phase to the gas phase. It is clear that the liquid phase RONS concentration (\({n}_{i, aq}\)) is determined by chemical reactions in the liquid phase \({\dot{\omega }}_{i, aq}\) and the surface molecular flux across the gas–liquid interface in the liquid phase \({\left.{{\varvec{\Gamma}}}_{{\varvec{i}},aq}\right|}_{s}\). The negative sign of the surface molecular flux \({\left.{{\varvec{\Gamma}}}_{{\varvec{i}},aq}\right|}_{s}\) indicates a species influx into the liquid phase from the gas phase. Here, the molecular flux normal to the interface \({\left.{{\varvec{\Gamma}}}_{{\varvec{i}},aq}\right|}_{s}\cdot {\varvec{n}}\) takes into account only the diffusion term \({\left.{{\varvec{\Gamma}}}_{{\varvec{i}},aq}\right|}_{s}=-{\left.({D}_{i}\nabla {n}_{i, aq})\right|}_{s}\) because of \({\left.{{\varvec{u}}}_{{\varvec{i}}}\right|}_{s}\cdot {\varvec{n}}=0\).
The flux \({\left.{{\varvec{\Gamma}}}_{{\varvec{i}},aq}\right|}_{s}\cdot {\varvec{n}}\) normal to the interface is rarely obtained in experiments [46, 47], and their direct measurement is hindered by technical challenges. The gas phase molecular kinetic flux \({\left.{\Gamma }_{i}\right|}_{gas}^{therm}\) can be used as the upper limit of the surface normal flux \(\left|{\left.{{\varvec{\Gamma}}}_{{\varvec{i}},aq}\right|}_{s}\cdot {\varvec{n}}\right|\) [48,49,50,51,52].
The symbols \({k}_{B}, {m}_{i}\), and \(T\) denote the Boltzmann constant, the molecular mass of species \(i\), and the gas temperature, respectively.
The characterization of the integrated RONS influx from the gas phase into the liquid phase and the molecular transfer rate (\(MTR\)) can be useful experimental scales, as discussed in a previous study [23]. The molecular transfer rate of species \(i\) (\(MT{R}_{i}\)) is the second term in Eq. (2) and is defined as follows:
-
Molecular transfer rate for long-lived RONS (\(MT{R}^{long}\))
For the long-lived reactive species RONSlong, the reaction term can be omitted if the liquid-phase reactions are negligible relative to molecular transfer rate (MTR) at the gas–liquid interface, where reactive species localization is pronounced owing to the nature of the surface influx. This assumption simplifies the integrated continuity equation as
where the superscript \(long\) indicates the long-lived species.
Defining the volume averaged concentration of species \(i\) in the liquid,
the mass balance equation for a liquid volume \({V}_{liq}\) is obtained as,
where \(-{\left.{\widetilde{\Gamma }}_{i,aq}^{long}\right|}_{s}\) is the effective surface normalized influx of the long-lived reactive species into the liquid phase.
It is clear that the \({\left.{\widetilde{\Gamma }}_{i,aq}^{long}\right|}_{s}\) and the liquid surface area \({S}_{liq}\) can simply enhance \(MT{R}^{long}\) and the accumulation rate of RONSaq (\(\frac{d}{dt}{\widetilde{n}}_{i,aq}^{long}\)), which can be estimated in an experiment. Notably, \({S}_{liq}\) can be significantly changed by deforming the gas–liquid interface, such as by gas bubbling. A larger \({S}_{liq}\) value typically magnifies long-lived RONS transport processes. It might be possible to assume that \({\left.{\widetilde{\Gamma }}_{i,aq}^{long}\right|}_{s}\) is identical to \({S}_{liq}\) in some cases, for example, when the surface flux reaches a diffusion limit \({\left.{\Gamma }_{{{i}}}\right|}_{gas}^{therm}\).
It is also important to mention that this equation is valid for opposite-directional transport, that is, molecular transport from the liquid phase to the gas phase. Long-lived species in the liquid phase can be lost to the gas phase through volatilization.
-
Molecular transfer rate for short-lived RONS (\(MT{R}^{short}\))
In contrast to RONSlong, the short-lived reactive species RONSshort exhibited a significant reaction loss process in the liquid phase, and its accumulation was prevented. This may allow the omission of the accumulation term \(\frac{d}{dt}{\widetilde{n}}_{i, aq}^{short}\) in Eq. (2). The integrated continuity equation for the short-lived species RONSshort can be expressed as follows:
This steady-state equation suggests that short-lived species are maintained steadily in the liquid phase by the surface RONS flux. Because \(MTR\) is often experimentally observable, the aforementioned definitions of \(MTR\) in Eqs. (7) and (8) can also be employed to experimentally classify species as either long-lived or short-lived.
Diffusion Loss of RONSaq to the Gas Phase
In “Diffusion Loss of RONSaq to the Gas Phase” and “Reaction Loss in the Liquid Phase” sections, the formulation is made for the RONSaq loss process, which also provides an interpretation of the opposite directional transport, that is, molecular influx into the liquid phase, as discussed in “Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface” section. Previous studies [49,50,51] noted that the molecular transport across the interface from the gas phase to the liquid phase is a diffusion-limited process and also inferred that the diffusion process, either in the gas or liquid phase, can dominate the net transport across the phase, depending on their physicochemical characteristics and initial conditions.
The loss mechanism for RONSaq can be broadly classified into two distinct scenarios: those primarily governed by diffusion and those primarily driven by reaction losses. In particular, for a low volume of water (e.g., microdroplets), the diffusion loss of RONSaq to the gas phase (volatilization) can be significant when the gas-phase RONS density drops drastically (e.g., shut-off or dilution of the plasma-generated RONS gas in contact with the liquid).
If the reaction loss is negligible, the diffusion loss can be formulated as:
Therefore, diffusion loss can be monitored by measuring the average concentration of the species in the liquid phase.
For simplicity, in this section, we discuss a static liquid sphere because it provides quantitative time and spatial scaling of the diffusion process with the aid of an analytical solution to gain insight into the diffusion loss process, which is similar to those in other coordinates.
Under the azimuthal and polar angle uniformity of the species concentration \({n}_{i}\left(r,t\right)\), one can find a simple and reasonable analytical solution to the continuity equation for the spherical coordinates using the zeroth spherical Bessel function of the first kind and the root of the boundary condition as follows:
where \({n}_{i}\) is a function of time and radial distance \(r\) from the center of a sphere with diameter \({d}_{drop}\).
This provides a solution in the form of an infinite series with a positive index of summation \(k\) as follows:
where the spatial distribution function \({R}_{k}\left(r\right)\), normalized by the liquid volume, is an orthogonal function of the following form:
which satisfies the following equations,
where \(\langle \cdot ,\cdot \rangle\) and \({\delta }_{kj}\) denote the inner product and the Kronecker’s delta function, respectively.
Time dependent function \({T}_{k}\left(t\right)\) is as given by
where \({T}_{k0}\) is the initial amplitude at \(t=0\) and \({\tau }_{k}\) is the decay time constant, which is obtained with the initial distribution of the species concentration \({n}_{i0}\left(r\right)\) as follows:
If \({n}_{0}\left(r\right)\) is spatially uniform, \({n}_{i0}\left(r\right)={\overline{n} }_{i0}\left(=const.\right)\)
This also provides a fraction of the molar number \({f}_{k0}\), which follows the individual decay time constant \({\tau }_{k}\) in the droplet.
This uniform initial concentration is valid for RONS, which decompose slowly in liquid water and are easily lost to the environment when the dissolved solution is rapidly exposed to the ambient environment.
A part of the analytical solution in the other coordinates can be found in textbooks, such as [52], and also yields similar \({\tau }_{k}\) and \({f}_{k0}\) with a different scale factor instead of \({d}_{drop}\).
Then, the diffusion loss rate obtained is shown below.
For a uniform initial concentration, \({\overline{n} }_{i0}\), the diffusion loss for a limited series up to \({k}_{max}\) diverges at \(t=0\); thus, the physical boundary should be considered. If the molecular kinetic limit in the gas phase is used, it can be formulated with the surface area of the droplet \({S}_{drop}\) as
where \({\overline{n} }_{i0,gas}\) is the gas-phase density of the species near the liquid surface, which is in equilibrium with the initial liquid-phase concentration, \({\overline{n} }_{i0}\).
With respect to the density unit conversion across the phases with Avogadro’s number \({N}_{A}\), the relation in Eq. (21) can be connected to Henry’s law constant \({H}_{i}^{cp}\) for species \(i\) as follows:
Table 1 shows that the diffusion coefficients in the liquid phase are approximately 2 × 10–9 [m2/s] while Henry’s law constant significantly varies. Therefore, \({k}_{max}\) can be estimated as a function of the droplet diameter \({d}_{drop}\) and Henry’s law constant, \({H}_{i}^{cp}\):
In most cases, an acceptable distribution was obtained using a \({k}_{max}\sim {10}^{3}\) value. A more detailed discussion of the boundary conditions for species influx from the gas phase to the liquid phase can be found in [49,50,51].
The analytical volume-averaged concentration can also be obtained independently, as follows:
The calculated decay of the volume-averaged concentration \({\widetilde{n}}_{i}\left(t\right)\) in a liquid droplet is shown in Fig. 3a for \({d}_{drop}\) = 50, 200, 1000 [μm] at \({D}_{i}=2\times {10}^{-9}\) [m2/s] and \({\overline{n} }_{i0}\) = 10 [μM]. As shown in Table 1, the diffusion constants in the liquid phase are similar for most known species. Thus, a similar density decay can be obtained for the species listed in Table 1. However, the decay of the mean concentration \({\widetilde{n}}_{i}\left(t\right)\) was largely affected by the droplet diameter \({d}_{drop}\). The decay time constants \({\tau }_{k}\) depicted in Fig. 3b for \(k\le 4\) illustrate that they pertain to a typical liquid droplet scale larger than 10 μm, extending to the measurement quartz cuvette scale of 10 mm.
Analytic solution of a diffusion loss in a liquid droplet. a Volume averaged mean concentration decay for various droplet diameter (\({D}_{drop}\)). b Decay time constants at \(k=1, 2, 3, 4\). Temporal decay with concentration distribution for c \({D}_{drop}\) = 200 μm and d \({D}_{drop}\) = 1000 μm
The time constant \({\tau }_{1}\) varies by six orders of magnitude, with a higher order of the decay time constant \({\tau }_{k>1}\) implying a significantly faster decay. The decay time constants and the molar fractions for \(k\le 5\), presented in Eqs. (17) and (20), respectively, are listed in Table 2. Figure 3c, d show the analytical solution for the spatial distribution of the concentration in the liquid droplet, \({n}_{i}\left(r,t\right)\). In Fig. 3c, the \({n}_{i}\left(r,t\right)\) distribution approximately follows the first-order distribution \({R}_{1}\left(r\right){T}_{1}\left(t\right)\), which indicates that the higher-order components have decayed at 0.4 s < \({\tau }_{1}\) (0.51 s). Figure 3c, d show that the impact of higher order is significant in the timescale \(t<{\tau }_{1}\). The mean concentration quickly decays within the first order decay time constant \({\tau }_{1}\) and then follows the first-order exponential decay. Approximately 40% of the liquid phase RONS, corresponding to \(\sum_{k>1}{f}_{k0}\), completely decayed within \({\tau }_{1}\) and only 22% of the initial mole’s amount of species \(t={\tau }_{1}\) remained in the droplet. Therefore, long-range transport, dominated by first-order decay that carries 61% of the dissolved species, can be approximated as follows:
Figure 3d shows that the species concentration decay was localized near the surface of a droplet with a diameter of 1 mm at the same timescale as shown in Fig. 3c. Therefore, the lifetime of the liquid-phase species is significantly extended, as shown in Fig. 3d.
A comparison of this diffusion loss timescale with the reaction loss timescale described in “Reaction Loss in the Liquid Phase” section gives a somewhat quantitative evaluation of the dominant loss process. In particular, for highly volatile species, particularly O3 and NO, diffusion can potentially play an important role in the liquid-phase reaction, generating short-lived species.
The diffusion process can be further complicated when convection is non-negligible for a large liquid volume and the fluid shear force becomes less significant. The applicability of diffusion transport can generally be characterized by a local Péclet number below 1 [63]; however, further studies on the plasma–liquid interface are required.
This diffusion loss analysis is analogous to gas dissolution in a liquid, as discussed in the previous section. The same time constant \({\tau }_{k}\) and molar number fraction \({f}_{k0}\) are shared for the surface flux; thus, those can be used to discuss the spatial uniformity and RONSaq localization in the liquid phase.
Reaction Loss in the Liquid Phase
The RONS loss mechanism in the liquid phase can be dominated by the reaction loss mechanism rather than the diffusion loss mechanism described in “Diffusion Loss of RONSaq to the Gas Phase” section, which assumes a negligible contribution of reactions in the liquid phase. The reaction loss can be described by neglecting \(MTR\) in Eq. (2) as an extreme case of molecular transport across the gas–liquid interface.
It should be mentioned that \({\dot{\omega }}_{i}\) represents the overall production and loss rates for species \(i\).
For a chemical reaction \(j\) among reactive species A, B, C, …
The reaction speed of the reaction \(j\) can be given by
where \({k}_{j}\) is the reaction rate constant and \(a,b,c \cdots\) are the stoichiometric coefficients.
While the complete expression for \({\dot{\omega }}_{i}\) can be found elsewhere, if the reaction \(j\) is the only reaction taking place in the liquid phase, the reaction loss for species A in the above reaction can be expressed as follows:
Note that this is the simplest reaction for loss, and in general, a variety of reactions for loss proceed simultaneously. Nevertheless, this expression can be helpful in interpreting experimental results under certain conditions, e.g., where reaction \(j\) plays a remarkable role as the key reaction for the loss of A and any other reactions can be negligible. The overall order of the reaction (\(a+b+c+\dots\)) is often two or higher in plasma-induced liquid-phase reactions. For a simple estimation of the reaction loss timescale, we discuss two types of second-order reactions and estimate the half-lives of the reactive species under the specified conditions.
The reaction speed for the second-order reaction with two of the same reactants (\(A+A\to \cdots\)) can be expressed using \({k}_{j}\) in M−1 s−1 as
where \(\left[A\right]\) is the concentration of species A, expressed as \({n}_{A0}\) in “Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface” and “Diffusion Loss of RONSaq to the Gas Phase” sections. For this disproportionate reaction at a given time, \(\left[A\right]\) can be analytically obtained as follows:
where [A]0 denotes the initial concentration of A. By extension, the half-life, \({t}_{1/2}\), is derived as follows:
Notably, this is the decay time for a simple single-species reaction system, and the actual reaction loss can be intense for plasma-induced reactions.
For a second-order reaction between two different reactants (\(A+B\to \cdots\)), provided that the concentration of one reactant is sufficiently higher compared to the other ([B] > > [A]), a pseudo-first order decay of \(\left[A\right]\) is obtained with the reaction speed \(v\le {k}_{j}\left[A\right]{[B]}_{0}={{k}_{j}}{\prime}[A]\) as follows:
The half-life time can be approximated as follows:
where [B]0 is the initial concentration of B.
The estimation of RONSaq lifetime using the above simple formula can be useful for the experimental design and identification of key RONSaq. Table 3 summarizes the half-life of two well-discussed reaction loss mechanisms: the self-decomposition/loss, including the disproportionation and autoxidation reactions in air-saturated water, and the typical reaction loss with the co-dissolved species shown in Fig. 1. The bimolecular chemical reactions selected for each RONSaq listed in Table 3 are empirically important in air and water plasma chemistry. In general, the plasma-induced reaction network is extremely complicated, and the key decomposition reaction strongly depends on various conditions (e.g., solution temperature, pH, and composition of coexisting RONSaq). These simplified estimations are accurate for dilute and uniform media and can be used to estimate the upper bound of the RONS lifetime in the liquid phase.
This also highlights the difficulty in categorizing RONS into “short-lived” and “long-lived” based on lifetime given by the reaction listed in Table 3 because the time constant is determined by the RONS composition and their own concentration. Under typical conditions (e.g., pH = 3–7, low species concentration) of air plasma-treated water chemistry, H2O2aq, NO2−aq, NO3−aq, and possibly O3aq are classified as long-lived RONSaq. However, except for NO3−aq, these have a limited self-decomposing lifetime, and the timescale threshold is ambiguous. Therefore, the definition from the mass balance presented in “Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface” section is used for the classification in this review.
The decomposition reaction loss in the presence of H2O2aq and HNO2aq included HOONOaq and HOONO2aq generation processes at pH < 4, which could be useful for various applications. This reaction loss and simultaneous RONS generation are very sensitive to solution pH and temperature [68]. Thus, the controlled transport of multispecies complexes is key for applications using HOONOaq and HOONO2aq [18], which provides important interpretations of the previous studies described in “Application Systems of Gas–Liquid Interface Transport of Air Plasma-Generated RONS for Plant Disease Control” section.
In particular, for highly volatile species (e.g., O3aq and NOaq), the volatilization loss under ambient conditions, as discussed in “Diffusion Loss of RONSaq to the Gas Phase” section, should be incorporated into the reaction losses for practical lifetime design. The timescales of self-decomposition, reaction loss with co-dissolved species, and volatilization loss can be compared with experiments, and some examples are introduced in “Plasma Effluent Gas Dissolved Solution (PEGDS) Spraying System” section.
Application Systems of Gas–Liquid Interface Transport of Air Plasma-Generated RONS for Plant Disease Control
Plasma Effluent Gas Exposure System
As discussed in “Integrated Mass Balance Equations for Reactive Species Transport Across Gas–Liquid Interface” section, the air plasma effluent gas can transport RONSgas to the liquid phase as RONSaq. RONS transport can induce liquid-phase reactions of the transported reactive species, which can generate short-lived RONSaq at the target liquid volume. Our previous study, aiming for antimicrobial effects with short-lived species generation, is an example (Fig. 4) of the characterization of the gas–liquid interface transport of RONS using MTRs [23, 25].

Reproduced from reference [23]
Plasma effluent gas exposure system. a Experimental setup for estimation of molecular transfer rates (MTRs) of long-lived RONS in humidified air plasma effluent gas. b Experimentally obtained molecular transfer rates (MTRs). c Suppression of conidium germination rate of a strawberry pathogenic fungus with the increase of the supplied liquid water flow into air DBD plasma to generate RONSgas. The inset figure indicates the relative germination rate against the exposure time \({t}_{e}\) to the plasma effluent gas at \({F}_{{\text{H}}2{\text{O}}}\) = 1500 μL min−1.
Water droplets contaminated with conidia or viruses of plant pathogens were exposed to humidified air plasma effluent gas, which transported gas-phase RONSgas into the contaminated liquid droplet. Figure 4a shows the humidified air plasma effluent gas generated by introducing liquid water into the air dielectric barrier discharge (DBD) plasma (\({F}_{{\text{H}}2{\text{O}}}\)). The densities of RONSgas were measured using Fourier transform infrared (FTIR) absorption spectroscopy, and volume-averaged concentrations of RONSaq \({\widetilde{n}}_{i}\) were measured using in-situ optical absorption spectroscopy (AS) [77, 78], Griess and Trinder’s reagent tests (RG) [79, 80], and ion chromatography at the end of a given exposure time [23, 40]. These data were analyzed using self-developed MATLAB applications. The circulatory reactive species trap (CRT) system shown in Fig. 4a was employed to rapidly homogenize the RONSaq concentration in the liquid volume \({V}_{liq}\) by forced mixing and gas bubbling, which also enhanced the effective liquid surface \({S}_{liq}\). Thus, the CRT system helps to magnify the MTRs of RONSaq, as discussed in Eq. (7).
Measurement of the volume-averaged RONSaq accumulation rate under negligible bulk reactions directly provides an estimate of \(\frac{d}{dt}{\widetilde{n}}_{i}^{long}\) and \(MT{R}^{long}\) using Eq. (7). The estimated MTRs shown in Fig. 4b were enhanced by \({F}_{{\text{H}}2{\text{O}}}\), which modified the composition of RONSgas. In CRT, the linear accumulation of NO3−aq, NO2−aq, and H2O2aq was observed, which is characteristic of “long-lived” RONS. Figure 4c shows that these MTR enhancements positively correlate with a reduction in the relative germination rate of the fungal conidia of a strawberry pathogen (C. gloeosporioides), which is normalized by the germination rate exposed to airflow. The inset of Fig. 4c shows that conidium germination was significantly suppressed within 10 s of exposure to humidified air plasma effluent gas.
Table 4 shows the estimated MTRs for CRT and for a liquid droplet with a low volume of 5 μL, which is the same as the conidium suspension liquid volume. Assuming the same RONSaq flux \({\left.{\widetilde{\Gamma }}_{i, aq}^{long} \right|}_{s}\) but with the different \({S}_{liq}\), the concentrations of NO2−aq and H2O2aq in the droplet, expected from CRT measurement, can be as high as 0.72 and 0.20 mM, respectively. However, the RONSaq composition in the liquid droplets likely differed from that in the CRT (Table 4). The NO3−aq accumulation for the droplet appeared to be a linear increase in 5 s sampling intervals, and the other RONS accumulation was not observed. Therefore, NO3−aq is the long-lived RONS, but NO2−aq and H2O2aq are not in the droplet.
Notably, no individual RONSaq in Table 4 exerted the conidium germination suppression effect shown in Fig. 4c at the given concentration. Table 5 shows the lethal concentrations (LC50) of individual RONSaq for C. gloeosporioides conidia. In addition, O3gas exposure alone was found to exhibit a negligible effect at the given exposure time and density and typically requires an exposure time longer than 120 s with 10 times higher O3gas density than that observed in the humidified air plasma effluent gas. Furthermore, the coexisting NO2−aq limiting reaction lifetime of O3aq (see Table 3) and the low Henry’s law constant in Table 1 also support the conclusion that the direct effect of O3aq has a negligible effect.
The well-characterized MTRs and discussion in “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” section help understand this conidium germination suppression effect and provide insights into short-lived RONS generation. The good agreement between the different measurement methods in Fig. 4b at different times supports the argument that the bulk reaction loss is negligible in CRT. Another explanation is based on the estimated RONS reaction loss in CRT. The estimated reaction lifetime \({t}_{1/2}\) of NO2−aq and H2O2aq according to Table 3 and 4 for CRT is longer than 5 × 104 s (estimated with [NO2−aq] = [HNO2aq]), sufficiently longer than a RONS accumulation time of \({t}_{e}=60\) s. This reaction lifetime estimation also suggests that the accumulation time \({t}_{e}\) for this case should not exceed 4 min when the linearly increased RONS drastically reduces \({t}_{1/2}\) to 2.1 × 103 s at \({t}_{e}\) = 300 s. Further details can be found in our previous studies [23, 40].
The prolonged duration required (30 s or longer) to measure the concentrations of RONS, as outlined in Table 4, coupled with the anticipated reaction loss time constant (\({t}_{1/2}\)) of approximately 60 s, suggests that both NO2−aq and H2O2aq are considerably depleted before the liquid droplet measurement. Consequently, deriving the MTRs from volume-averaged concentrations of low-volume liquids becomes challenging. This circumstance underscores a significant reaction loss process for NO2−aq and H2O2aq, indicating the generation of antimicrobial HOONOaq and HOONO2aq within these low-volume liquids. The germination suppression effect in Fig. 4c appears to correlate with the MTR enhancement with \({F}_{{\text{H}}2{\text{O}}}\) in Fig. 4b, which also indicates the correlation with reaction loss processes for NO2−aq and H2O2aq and HOONOaq and HOONO2aq generation under acidic pH = 0 − 4.4 as follows:
Notably, reaction (R1) is frequently described as a second-order reaction (R1’); however, its applicable pH range is limited.
The HOONO2aq can be subsequently generated in the liquid as follows:
The above HOONOaq and HOONO2aq generations qualitatively explain the improvement in the conidium germination suppression effect shown in Fig. 4c based on the higher MTRs of RONSaq. However, the bulk chemistry of HOONOaq and HOONO2aq generated using the estimated MTRs could not entirely explain the antimicrobial effects shown in Fig. 4c. A post-mixture of the plasma effluent gas-exposed droplets with the conidium suspension partially suppressed conidium germination; however, its suppression rate was significantly lower than that of direct exposure to plasma effluent gas [23].
The localization of RONSaq of HOONOaq and HOONO2aq in the droplets during humidified plasma effluent gas exposure can explain the germination suppression effect in Fig. 4c. The scale of localization near the surface of a millimeter-sized liquid sphere can be limited by the diffusion process in the liquid phase. The diffusion loss time constant in Fig. 3b of \({\tau }_{1}\) ~ 13 s is analogously shared in the RONS dissolution into the liquid phase. The first-order time constant \({\tau }_{1}\) indicates the timescale of the higher-order decay; thus, the timescale of the long-lived RONSaq localization near the liquid surface can be characterized with \({\tau }_{1}\). Figure 4c shows that the germination suppression effect is achieved within 10 s, which is less than the diffusion timescale \({\tau }_{1}\). Therefore, RONSaq must be significantly concentrated near the liquid surface compared with the volume-averaged RONSaq in the CRT, and the high-order reaction loss mechanism, which concurrently generates HOONOaq and HOONO2aq, must occur significantly under the given conidium suspension liquid exposure to the humidified air plasma effluent gas. Due to the self-decomposition lifetime of HOONOaq (1 < \({\tau }_{1}\)), their diffusion into the depth of the liquid is limited. In contrast, HOONO2aq, generated via pseudo-fourth-order reactions R1–R3, appeared to have a sufficient lifetime to spread out within the liquid droplet.
In Fig. 4c, a small decrease in the germination suppression was found at \({F}_{{\text{H}}2{\text{O}}}\) = 0, where the MTRs were significantly low except for that of NO3−aq and high-order reactions barely occurred. The near-surface reaction of N2O5 is proposed [40], which is known to produce NO3−aq through dissolution [76] and forms intermediate short-lived species, namely nitronium ion \({{\text{NO}}}_{2{\text{aq}}}^{+}\), solvated nitronium ion \({{\text{H}}}_{2}{\text{O}}\cdot {{\text{NO}}}_{2{\text{aq}}}^{+}\), and the contact ion pair \({\left[{{\text{NO}}}_{2}^{+}\cdot {{\text{NO}}}_{3}^{-} \right]}_{{\text{aq}}}\). The reaction formula is as follows:
The \({{{\text{NO}}}_{2}^{+}}_{{\text{aq}}}/{{{\text{H}}}_{2}{\text{O}}\cdot {{\text{NO}}}_{2}^{+}}_{{\text{aq}}}\) is known to react with H2O2aq, as in R3 [68]. The experimental resolution of this intermediate was limited; however, the reaction pathways near the liquid surface have been numerically studied [81].
The HOONO2aq production process R3 with N2O5 during plasma effluent gas exposure can be summarized as a second-order reaction at the gas–liquid interface, as follows:
This mechanism is also valid for air plasma effluent gas bubbling through \({{\text{H}}}_{2}{{\text{O}}}_{2{\text{aq}}}\), which enhances its antibacterial effect [82]. Considering the reactivity of NO2+aq with the solvent H2Oaq, reaction R5 should be significant only in the thin layer near the gas–liquid interface and should be treated as a short-lived species supply to the gas–liquid interface.
Our previous study experimentally demonstrated that N2O5gas exposure to the liquid surface exhibited “short-lived” RONS characteristics with the CRT system, and the liquid phase reactivity was represented by Eq. (8) [40]. The NO3−aq measurements in CRT without liquid phase reactants (\(MT{R}_{NO3-}^{long}\)) and the RONSgas composition measurements on N2O5gas and HNO3gas allow the estimation of \(MT{R}_{NO2+}^{short}\) as follows:
where \(MT{R}_{HNO3}^{long}\) represents the contribution of HNO3gas to \(MT{R}_{NO3-}^{long}\) and it is important to mention again that this equality holds unless solution contain any scavengers against NO2+aq. Thus, the long-lived RONSgas transport to the liquid can activate bulk reactions, surface-localized reactions, and near-surface or interfacial reaction pathways to generate short-lived RONSaq.
Plasma Effluent Gas Dissolved Solution (PEGDS) Spraying System
The liquid target exposed to the plasma effluent gas can contain various dissolved RONSaq values, as discussed in “Plasma Effluent Gas Exposure System” section. In this section, spraying water exposed to plasma effluent gas, hereafter referred to as the plasma effluent gas dissolved solution (PEGDS), transports the dissolved RONSaq to the target.
PEGDS spray transport to the target requires a sufficiently long RONSaq self-decomposition lifetime to prevent decay by the target and manage the diffusion loss described in “Diffusion Loss of RONSaq to the Gas Phase” section, as shown in Fig. 3. Figure 5a shows a schematic view of the device spraying PEGDS onto the C. gloeosporioides conidium suspension liquid for the antimicrobial effect to suppress plant pathogens, as described in “Plasma Effluent Gas Exposure System” section. The PEGDS in Fig. 5a mainly transports O3aq, unlike that in “Plasma Effluent Gas Exposure System” section, whose lifetime and concentration control in the target liquid are required to prevent damage to plants as a side effect [41]. PEGDS was generated and sprayed through a two-fluid spray nozzle to transport the dissolved O3aq, which has a low Henry’s law constant (Table 1). The immediate spraying of PEGDS onto the target minimized any loss.

Reproduced from reference [24]. d The decay of O3aq with a known initial concentration of 23 μM and the nominal droplet diameter of 280 μm, with analytical solutions described in “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” and “Plasma Effluent Gas Exposure System” sections
Plasma effluent gas dissolved solution (PEGDS) spraying system. a Schematic of experimental setup for PEGDS spraying system. b Decay of O3aq concentration in the sprayed solution collected at 9 cm downstream from the nozzle exit and c relative germination rate as a measure of anti-microbial effect in sprayed PEGDS collected at 9 cm downstream with 10 s delay for mixing with the conidium suspension liquid.
Figure 5b shows the O3aq concentration in the PEGDS collected at the target (9 cm downstream from the nozzle exit), which decayed with retention time before the O3aq concentration measurement. The PEGDS was sprayed with air plasma effluent gas and oxygen plasma effluent gas with similar O3gas densities of 4.1 × 1016 and 4.5 × 1016 cm−3, respectively [24]. The air PEGDS resulted in 30% less O3aq concentration below 1 μM in Fig. 5b. This was reflected in the relative germination rates shown in Fig. 5c. The germination suppression effect for both PEGDSs was similarly correlated with the measured O3aq concentration [24], which indicates that O3aq, which is the major RONSaq in both PEGDSs, plays a key role in the germination suppression effect, as shown in Fig. 5c.
In terms of the agricultural application of PEGDS with O3aq, a greater spray distance is desired but was limited to 10 cm, as shown in Fig. 5b, regardless of the spray time for PEGDS. This suggests that the loss mechanism is significant at certain distances. Based on the O3gas density and Henry's law constant, the initial concentration of O3aq can reach 19 μM; however, the measured concentration collected at the nozzle exit was approximately 5 μM [24]. This could be due to the insufficient dissolution time (30 ms) used in the two-fluid spray nozzle, reaction loss during the dissolution process in the spray, or higher-order volatilization loss before the measurement.
Figure 5b indicates that the O3aq concentration decays from 5 to 1 μM at 9 cm downstream from the nozzle exit. As shown in Fig. 5b, O3aq concentration is depleted in the collected liquid over a period of approximately 200–500 s, which is longer than the time of flight of the sprayed PEGDS droplet \({\tau }_{O3}^{f}\) (ranging from 10 to 100 ms) but shorter than the self-decomposition lifetime of ~ 103 s in Table 3.
As shown in Fig. 5a, O3aq in PEGDS was sprayed into ambient air after passing through a nozzle. As a result, the liquid droplets experience a sudden decrease in the O3gas density at the gas–liquid interface, which causes volatilization loss, as discussed in “Diffusion Loss of RONSaq to the Gas Phase” section. Additionally, it was not possible to avoid the volatilization loss of the collected PEGDS downstream, whose thickness scale was only a few millimeters. The first-order decay time constant \({\tau }_{1}\) in Eq. (17) and Fig. 3b ranges from 10 to 100 s, which is qualitatively in good agreement with the decay in Fig. 5b. The O3aq concentration decay after passing through the nozzle (from 5 to 1 μM) during the time of flight \({\tau }_{O3}^{f}\) depends on the diameter of the sprayed droplets, which was not measured. Assumption \({\tau }_{O3}^{f}\cong {\tau }_{1}\) gives the estimated typical droplet diameter (\({D}_{drop}^{typical}=2k\pi \sqrt{{\tau }_{O3}^{f}{D}_{i}}\)) as 28–90 μm, which is a reasonable droplet scale.
The lifetime control in \({\tau }_{O3}^{f}\) is key for agricultural applications as it allows the scaling of the anti-microbial effect without damaging the plants. Further studies on the O3aq decay within \({\tau }_{O3}^{f}\) have been carried out using an O3-dissolving bath to equilibrate O3aq with O3gas and a one-fluid spray nozzle to simplify the ambient gas condition. The nominal droplet diameter of the one-fluid spray nozzle is 280 μm, and the O3aq concentration in the bath was 23 μM. The decay of the volume-averaged concentration was compared with the droplet speed, which was assumed to be constant at 1 m/s.
The black dots in Fig. 5d show the measured O3aq concentration collected at a given distance, and the solid black curve indicates the analytical solution of the volume-averaged concentration \({\widetilde{n}}_{i}\left(t\right)\). One can find that the first-order solution \({\widetilde{n}}_{i, 1}\left(t\right)\) (dashed green curve) gives a good approximation for \({\widetilde{n}}_{i}\left(t\right)\) at distances > 50 cm, corresponding to \(t>{\tau }_{1}=0.507\;\text{s}\) in Eq. (27). In Fig. 5d, the decay trend of the measured volume-averaged concentration is closer to that of the approximated analytical solution \({\widetilde{n}}_{i, 1}\left(t\right)\), but the measured concentration is lower by a factor of 0.7.
The dashed red curve in Fig. 5d shows \({f}_{10}{\widetilde{n}}_{i}\left(t\right)\), which represents the high-order decay loss of O3aq and approximately follows the measured volume-averaged O3aq concentration. The high-order loss, which is responsible for near-surface RONSaq volatilization, can occur not only during the time of flight of the sprayed PEGDS but also when it is collected due to the mixing of the solution. The relatively good agreement between the measured concentration and \({f}_{10}{\widetilde{n}}_{i}\left(t\right)\) can indicate that some fraction of analytical solution \({\widetilde{n}}_{i}\left(t\right)\) is lost during the PEGDS collection but less than \(\sum_{k=2}^{\infty }{f}_{k0}\sim 0.4\) or the fraction factor \({f}_{k0}\) in Table 2 for sphere is not accurate for 1 mL of PEGDS on a tray. This quantitative agreement indicates that PEGDS transport by spraying is predictable and controllable during volatilization.
Figure 5b shows another factor that controls PEGDS concentration and lifetime. The concentration of the air PEGDS was lower than that of the O2 PEGDS. This difference was explained by the co-dissolved NO2−aq concentration of ~ 0.5 μM, which caused an O3aq reaction loss of \({t}_{1/2}\) ~ 3.8 s. This O3aq reaction loss has been verified with artificially added NO2−aq, showing a negligible anti-microbial effect (data not shown). This additional reaction loss can reasonably explain the lower O3aq for air-PEGDS and the reduced germination suppression effect, as shown in Fig. 5b, c. These different loss mechanisms provide a degree of freedom to separately modulate the dosage and lifetime of O3aq at the target, and future studies can be extended to other RONSaq transports.
Air Plasma-Irradiated Solution (APIS) Spraying System
The gas–liquid interface transport models presented in “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” section are not limited to modeling the dissolution of RONSgas in the plasma effluent gas to form RONSaq, as described in “Plasma Effluent Gas Exposure System” and “Plasma Effluent Gas Dissolved Solution (PEGDS) Spraying System” sections, but are also applicable to the gas–liquid interface in direct contact with the plasma. In this section, a plasma device using a high-speed liquid jet in direct contact with the plasma, namely an air plasma-irradiated solution (APIS), is presented to generate and transport RONSaq for the same agricultural applications.
In the context of agriculture, it is desirable for the plasma process to exhibit the following characteristics: utilization of materials present in the agricultural field, scalability, absence of adverse effects, and the production of harmless residual byproducts. The spray transport of PEGDS generated from air and water demonstrated the scalability of the antimicrobial effect, as discussed in “Plasma Effluent Gas Dissolved Solution (PEGDS) Spraying System” section. However, O3 can cause side effects [41]. Short-lived RONSaq generation, such as HOONOaq and HOONO2aq discussed in “Plasma Effluent Gas Exposure System” section, would be practically useful because their final product is NO3−aq, a nitrogen nutrient for plants. However, one of the drawbacks of using long-lived RONSgas is the loss of RONSgas to the environment.
Figure 6a shows the developed APIS device aimed at the efficient generation and transport of HOONOaq and HOONO2aq, whose self-decomposition lifetimes range from 1 to 100 s depending on the solution pH in Table 3. A condensed liquid fertilizer solution (KNO3aq) of 100 mM at pH 3 was pumped through an electrically grounded stainless tube with a 0.25 mm inner diameter (ID) at the axis of the quartz discharge tube with a 4 mm ID. The conductive liquid jet grounded through the stainless-steel tube ensured liquid contact with the plasma generated by the high-voltage electrode on the outer surface of the quartz tube. As shown in Fig. 6a, the liquid jet forms a laminar liquid column. The laminar liquid column length, which becomes unstable downstream depending on the solution flow rate \({F}_{sol}\), can be expected to represent the electrical connection to the ground and is therefore related to the discharge coupling power and plasma–liquid contact surface area.
Air plasma irradiated solution (APIS) spraying system. a Schematic of experimental setup for APIS with an inset photo of the liquid jet flow visualization. b RONSgas’s densities in the effluent gas, c RONSaq’s concentrations and the length of laminar liquid column, and d Relative germination rate of a pathogen conidium as a function of the solution flow rate (Fsol). e Relative germination rate at Fsol = 10 mL/min as a function of the transport distance with the calculated time of flight. The conidium germination rates in (d, e) are used as a measure for anti-microbial effect in the splayed APIS. At 9 cm downstream from the quarts tube exit, the conidium suspension was placed and the sprayed APIS was collected in (c, d)
Figure 6b shows the RONSgas in the effluent measured after extraction into the FTIR gas cell. As the flow rate increased, the composition of RONSgas changed from NOgas-dominant to NO2gas-dominant and eventually became O3gas-dominant. The measured laminar liquid column length as a function of the solution flow rate \({F}_{sol}\) is shown in Fig. 6c. The length of the laminar liquid column gradually increased from \({F}_{sol}\) = 4 mL/min and then saturated at approximately 8 mL/min. The liquid column gradually became unstable at \({F}_{sol}\) > 14 mL/min, which coincided with the gas composition change to O3gas-dominant in Fig. 6b. Figure 6c also shows the volume-averaged concentrations of H2O2aq, measured with Trinder’s reagent (RG) and titanium oxysulfate (TiOSO4) [83, 84], and HNO2aq/NO2−aq, measured with Griess-reagent (RG). The concentration decay of H2O2aq and HNO2aq/NO2−aq due to the solution flow rate increase at \({F}_{sol}\) < 10 mL/min can be interpreted as the dilution effect. Unlike PEGDS in Fig. 4, the volume-averaged concentration of H2O2aq is clearly higher than that of NO2−aq in Fig. 6c.
Figure 6d shows the germination rate of the conidia as a function of \({F}_{sol}\). While none of the individual RONSaq at the measured concentrations showed a germination suppression effect, APIS clearly suppressed the germination rate in the \({F}_{sol}\) range from 7 to 14 mL/min, where laminar flow is formed in the discharge volume. At a \({F}_{sol}\) of approximately 12 mL/min, the discharge and RONS in both phases in Fig. 6b, c sometimes exhibit a significant jump. This jump in RONS and discharge resulted in uncertainty in their trends (\({F}_{sol}\) > 12 mL/min), which was interpreted as a plasma–liquid interaction.
Figure 6e shows that at 10 mL/min, the conidium germination suppression effect gradually deteriorated as the distance of the conidium suspension target from the discharge tube exit increased. Using the calculated time of flight, it can be estimated that the antimicrobial effect is lost in the range of 20–50 ms. Because none of the RONSaq remained downstream, which represents the germination suppression effect, as demonstrated in Fig. 6e at 300 mm, this germination suppression effect near the device exit was likely exerted by short-lived RONSaq.
The key short-lived RONSaq representing the germination suppression effect in Fig. 6d may be HOONOaq in the APIS in Fig. 6. Its half-life at the measured liquid temperature ~ 55 °C at 10 mL/min is approximately 30–40 ms from the decay constant and activation parameters [68, 85, 86]. This value is very close to the calculated time of flight, ranging from 20 to 50 ms. However, none of the measured long-lived RONSaq in Fig. 6c, which is the precursor of HOONOaq, correlates well with the germination suppression effect in Fig. 6c. Comparing Fig. 6b, d, the germination suppression effect appears to correlate with NO2gas; however, in the liquid phase, the HNO2aq/NO2−aq is reduced with \({F}_{sol}\). Although it is difficult to conclude if there is a significant reaction loss for HNO2aq/NO2−aq before the measurement, the observed conidium germination suppression effect in Fig. 6d cannot be sufficiently explained by the bulk reactions of R1 to R5.
The focus can be diverted to the higher generation of H2O2aq than NO2−aq in Fig. 6c compared to the PEGDS prepared by humidified plasma effluent gas in Fig. 4b. A number of studies [8, 15] have shown that H2O2aq can be formed at the plasma–liquid interface through disproportional reactions of OHaq and HO2aq/O2−aq near the gas–liquid interface, which supports the presence of more H2O2aq than NO2−aq in Fig. 6c compared to the PEGDS in Fig. 4. This near-surface H2O2gas generation indicates highly localized OHaq/HO2aq/H2O2aq in the acidic APIS (pH = 3), as shown in Fig. 6. This is advantageous for inducing higher-order reactions (R1–R5). In addition, OHaq has been suggested to entrain volatile NOgas/NO2gas into the liquid phase [37, 38] and contribute to the generation of HOONOaq through the following pseudo gas–liquid interface reaction:
Volatile NOaq/NO2aq may exhibit significant reaction loss in the liquid phase near the interface due to highly localized OHaq and, therefore, may allow the definition of \(MT{R}_{NO}^{short}\)/\(MT{R}_{NO2}^{short}\) in terms of MTR discussion with Eq. (8). Equation (8) indicates that the reaction loss due to localized OHaq determines the characteristics of either \(MT{R}^{short}\) or \(MT{R}^{long}\). This can be enhanced by increasing the effective surface area corresponding to the length of the laminar liquid column.
The byproducts of the reactions of NOaq/NO2aq with OHaq are HNO2aq and HNO3aq. While the volume averaged concentrations of HNO2aq/NO2−aq and H2O2aq are decreasing with increasing \({F}_{sol}\) in Fig. 6c, the HNO2gas density was increased to a detectable level in Fig. 6b when the laminar column was well-maintained and the H2O2gas density was kept below the detection limit. A portion of the HNO2gas density in Fig. 6b can be interpreted as volatized near-surface HNO2aq, whose Henry’s law constant in Table 1 is much lower than that of H2O2aq. This is consistent with the observation that HNO2gas was not present in the series of experiments in “Plasma Effluent Gas Exposure System” and “Plasma Effluent Gas Dissolved Solution (PEGDS) Spraying System” sections, where localized OHaq generation was not expected. Although H2O2aq and HNO3aq may also be localized near the interface, it is difficult to detect the volatized portion because their Henry’s law constants are high, as shown in Table 1.
The above phenomenological conjunction across the phases that might exhibit the pseudo-interfacial reaction between NOgas/NO2gas and localized OHaq/HO2aq/H2O2aq, such as (R6), becomes false at high flow rates because in Fig. 6c, the gas phase NO2gas is significantly reduced at \({F}_{sol}>\) 14 mL/min. The reduction in the laminar column at \({F}_{sol}\) > 16 mL/min may coincide with the gas temperature drop near the liquid surface and the solution temperature drop, likely owing to the reduction in the channels bridging the electrical current through the liquid surface. Because higher temperatures assist nitrogen dissociation [87], typically observed as nitrogen oxide that promotes O3gas decomposition, the temperature drop coincidentally occurring with the laminar column collapse can reduce the NO2gas density.
The pseudo-interfacial reaction in the APIS was tested by introducing air plasma effluent gas with the composition given in Table 6 instead of using air. Table 6 shows that the air plasma effluent gas prepared prior to the APIS device contained a similar density of NO2gas, as shown in Fig. 6b. Figure 6d clearly shows an improvement in the germination suppression effect upon increasing \({F}_{sol}\) > 7 mL/min, which supports our argument that the near-surface pseudo-reaction between OHaq/HO2aq/H2O2aq and NOgas/NO2gas to form HOONOaq may play an important role in suppressing germination.
Although the localization of OHaq and the NOgas/NO2gas uptake by OHaq have been discussed over the past several years [37, 38], to the best of our knowledge, these models and ideas have not been validated experimentally. Further quantitative studies on the near-surface liquid-phase chemistry of short-lived RONSaq generation, particularly actual \(MT{R}_{OH}^{short}\) measurements, are extremely important to resolve these phenomena and to design an APIS that can satisfy all requirements for agricultural applications.
Selectively Synthesized Dinitrogen Pentoxide Gas Exposure System
As discussed in “Plasma Effluent Gas Exposure System” section, N2O5gas could potentially play key roles in enhancing the antibacterial efficacy of the air plasma effluent gas in terms of the near-surface reaction through intermediate species (e.g., \({{\text{NO}}}_{2{\text{aq}}}^{+}\), \({[{{\text{NO}}}_{2}^{+}\cdot {{\text{H}}}_{2}{\text{O}}]}_{{\text{aq}}}\), \({[{{\text{NO}}}_{2}^{+}\cdot {{\text{NO}}}_{3}^{-}]}_{{\text{aq}}}\), \({{{\text{N}}}_{2}{{\text{O}}}_{5}}_{{\text{aq}}}\)). The intermediate species have been experimentally confirmed by a competing reaction of N2O5gas solvation to generate NO3−aq with p-hydroxyl-phenyl-acetate (p-HPA) at pH = 11 [40], which suppressed approximately 50% of NO3−aq generation. The unique behavior and importance of N2O5 chemistry at the gas–liquid interface have also been reported in inorganic chemistry and atmospheric chemistry [68, 76, 81, 88, 89]. However, N2O5gas has not been extensively studied or utilized because of the difficulties associated with its conventional synthesis and storage. Conventional N2O5 synthesis requires multiple hazardous raw materials, demanding the utmost care in its handling due to its high sensitivity to heat and moisture.
In our previous work, the dominant route of N2O5gas generation in the air plasma effluent gas was found to be the oxidation of NOgas/NO2gas with O3gas, which was determined through a stoichiometric analysis of RONSgas chemistry [40]. This quantitative analysis of RONSgas chemistry provides a pathway for the development of a high-density, highly selective, and continuous process for generating N2O5gas exclusively from air with a power consumption of less than 100 W [43]. Briefly, as shown in Fig. 7a, air through a gas dryer, typically below the − 60 °C dew point, was continuously supplied to two plasma reactors [a high-temperature (HT) DBD plasma reactor and a low-temperature (LT) DBD plasma reactor] at a fixed flow rate of 1 L/min each. The HT plasma reactor, which was designed to increase the gas temperature with discharge coupling power and heat recovery, primarily generated nitric oxide (NOgas) and nitrogen dioxide (NO2gas), whereas the LT plasma reactor generated O3gas. The subsequent mixing of these plasma effluent gases in a perfluoroalkoxy alkane (PFA) tube to significantly consume NOgas/NO2gas and O3gas in an air flow reached a reaction time of up to approximately 30 s, resulting in continuous N2O5gas generation at the tube exit with a selectivity of up to 20 at a density of approximately 300 ppm. Furthermore, this system can provide not only selective N2O5gas gas but also NOgas/NO2gas- or O3gas-rich gas simply with electric switching for the HT and LT plasma reactors, as shown in Fig. 7b. Recent studies confirmed that successive dilutions with dry air enable gas density control with a similar composition ratio. This RONS control technology for air plasma chemistry provides a new approach for accessing the RONSgas supply to the gas–liquid interface, including short-lived RONSaq.
Selectively synthesized dinitrogen pentoxide gas exposure system. a Experimental setup for on-site N2O5gas generation with high selectivity, exclusively from air and electricity. b RONSgas’s densities with electric switching for the HT and LT plasma reactors. c Experimental schematic and d experimental results of a Qβ phage inactivation experiment by N2O5gas and O3gas selectively generated the air plasma system. a, b are reproduced from reference [43]. Copyright (2020) American Chemical Society
Reactivity at the gas–liquid interface has been experimentally demonstrated by exposing the virus and plants to plasma-generated N2O5gas. Figure 7c shows a schematic of a bacteriophage Q beta (Qβ phage) inactivation experiment using the selectively generated N2O5gas and O3gas [11]. The Qβ phage suspended in distilled water (DW) or phosphate buffer (PB) at 108 PFU, atomized to typically 5 μm by a nebulizer, was mixed in a gas tube with a downstream collector with 100 mM PB solution for up to 15 min, controlled by the flow valves. The residence time of the virus mist in the mixing gas tube was estimated to be below 3 s. Figure 7d shows that the PFU with the N2O5gas-rich gas and the O3gas-rich gas were found to be below the detection limit, effectively inactivating the Qβ phage in the liquid mist where the gas–liquid interface reaction can be pronounced. In addition, the inactivation effect of the N2O5gas-rich gas on the Qβ phage in PB was significant, similar to that on the Qβ phage in DW, whereas the buffering capacity of PB to counteract the acidification by the N2O5gas dissolution was still present in Fig. 7d. This virus inactivation effect in the mist could be interpreted as the contribution of the N2O5gas-derived intermediate species at the gas–liquid interface instead of a simple acidification effect.
Plant disease control includes not only the sterilization and inactivation of plant pathogens but also the regulation of plant immunity, which is the innate capacity of plants to defend against and resist pathogenic invasions. Plant activation of N2O5 for plant immunity was discovered by a sequential testing process. Arabidopsis thaliana exposure to the N2O5gas-rich gas was followed by pathogen inoculation of the exposed plants [35]. Gene expression analysis indicated the activation of signaling pathways for jasmonic acid (JA) and ethylene (ET), which are important phytohormones involved in plant immunity. These results indicated that N2O5gas exposure induces plant activation. Thus, advancements in air plasma chemistry control provide new opportunities for exploration, and we are presently engaged in further experimental investigations using N2O5.
Future Prospects on Gas–Liquid Interfacial Air Plasmas
The measurement and estimation of the transport mechanisms of RONS have largely contributed to the understanding of GLIP and the associated short-lived RONSaq generation and transport mechanisms, which is numerically expected [90]. As discussed in “Air Plasma-Irradiated Solution (APIS) Spraying System” section, the distribution of short-lived RONSaq (e.g., OHaq, HO2aq, and NOaq) cannot be characterized experimentally because of their high reactivity and nonuniformity.
A potential approach to resolving the short-lived RONS in the experiment is to introduce liquid motion through the plasma, as described in “Air Plasma-Irradiated Solution (APIS) Spraying System” section. Liquid jets [91, 92] and liquid droplets [49,50,51] have demonstrated potential for establishing quantitative discussions based on experiments and numerical/analytical models. This gas–liquid biphasic flow reactor has been intensively studied in atmospheric chemistry [48]. The sub-millimeter scale of the liquid would be advantageous for resolving near-surface phenomena in terms of a low Reynolds number and Péclet number. This indicates that the reactive species distribution in the liquid can be dominated by diffusion transport with a minimum convection effect, and some simplification in the numerical modeling can be used to study highly localized, short-lived RONSaq.
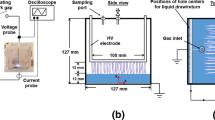
Recently, a helium-GLIP with a high-speed liquid column jet flow through a helium-APP was used to experimentally detect OHaq decay [91]. DW flow was ejected from a grounded stainless tube with an inner diameter of 0.13 mm into the helium APP along its central axis at an estimated speed of 12.6 m/s. The liquid contact with the helium APP, powered by applying a high AC voltage with a coupling power of approximately 0.4 W in Fig. 8a, was bounded by the ground electrode underneath a Teflon plate with a hole (φ = 0.8 mm). The cross-sectional average concentration of OHaq ([OH]csa) was determined experimentally using terephthalate anion (TA2−), which reacts with OHaq to generate a highly fluorescent 2-hydroxylterephthalate ion (HTA2−). A TA reagent flow at approximately 1.1 mL/min from a 0.11 mm PEEK tube collided with the water flow, enabling collisional mixing and trapping of OH at a specific distance (dg) from the Teflon plate. Figure 8b illustrates the experimental decay plots of [OH]csa, with a time resolution of approximately 0.08 ms, attributable to the high flow speed of 12.6 m/s. Figure 8b shows the calculated decay of [OH]csa with the disproportional reaction in Table 3, assuming (A) a uniform distribution and (B) a highly surface-localized distribution. A better agreement of model (B) with the experimental [OH]csa decay of 0.1 ms indicates that OH is localized near the gas–liquid interface. Further precise studies with both experiments and numerical modeling can provide the surface-localized OH and \(MT{R}_{OH}^{short}\) and may allow the establishment of those with other RONS, enabling the direct quantification of \(MT{R}^{short}\) or fluence [90].

(Reproduced from reference [91]. Copyright (2021) The Japan Society of Applied Physics
a Schematic drawing for helium-GLIP with high-speed water jet flow. b The cross-sectional average concentration of OHaq ([OH]csa) as a function of the distance of the collisional-mixing point from the PTFE hole exit (dg). The black line and the blue line are calculation results assuming a uniform cross-sectional distribution of OHaq and a OHaq consumption only by a 2nd order recombination for [OH] = 88 and 1800 nM at dg = 1 mm, respectively.
As briefly described in “Introduction” section, the simplification of the gas–liquid interface highlights the reaction chemistry of RONSaq transport across the phases; however, the motion of the liquid interface should also be investigated to develop a comprehensive and descriptive RONS transport model across the biphasic interface. Recent pioneering studies have discussed the importance of surface charge [93,94,95] and surface tension [96, 97], which are key forces that directly act on the liquid surface. The surface charge at the liquid surface, resulting from the transfer of charged species, exerts Coulomb forces on the gas–liquid interface. The modification of the localized force balance with the fluid shear force and surface tension may induce a significant secondary flow near the gas–liquid interface, which would affect the reaction chemistry. This is important because the liquid surface motion and morphology can influence the molecular transport phenomena \(MT{R}^{short}\) and \(MT{R}^{long}\), which are concurrently influenced by the surface localization of RONSaq (e.g., NOgas/NO2gas uptake via the pseudo-interfacial reaction, as discussed in “Air Plasma-Irradiated Solution (APIS) Spraying System” section). This hypothetical interaction mechanism, associated with the molecular transport phenomena across phases, represents the complex nature of GLIP and signifies a critical gap in the integration of knowledge regarding GLIP, which is essential for the design of targeted RONSaq delivery.
Reduced complexity would also help untangle the interaction mechanisms associated with molecular transport through a moving liquid surface. A liquid column jet and water droplet in contact with the gas/plasma phase can essentially suppress the secondary flow near the surface when its scale is sufficiently small, as indicated by the Reynolds number Re ~ 1. When in contact with a small-scale liquid, these GLIPs may suppress the higher-order spatial flow modes that are commonly observed in turbulence. This simplified effect of the surface motion on the interaction is attributed to the reduction in the degrees of freedom of the surface motion. Furthermore, the small, symmetrical surface structure allows for additional simplifications. It is reasonable to assume azimuthal uniformity of the surface flux for a liquid column jet with a diameter below 1 mm in uniform helium plasma [92], and azimuthal and polar uniformity for a small liquid droplet.
As depicted in Fig. 9, in conjunction with temporally and spatially controlled \(MTR\) with independently accumulated surface charges, such as nanosecond pulse discharge plasma with a biased voltage [42, 98], the reduced-mode liquid surface may experimentally allow the resolution of the interaction effect on molecular transport across the phase. For this purpose, measurements of the RONSgas density distribution, flow velocity, liquid surface morphology, electric field, and surface charge are currently ongoing in addition to the liquid-phase measurements discussed in this review. Notably, such a small-scale and limited fluid dynamical mode will significantly reduce the numerical simulation costs; therefore, it would allow direct comparison with the experimental data, which is beneficial for developing a descriptive model of the molecular transport phenomena in GLIP. These developments can be adopted to achieve completely controlled biphasic RONS transport.
Conclusion
In “Simplified Modeling of RONS Transport Across Gas–Liquid Interface” section, we introduced an integrated mass balance model for biphasic RONS transport across the plasma gas–liquid interface. This model encompasses insights into species lifetime concerning molecular transport across phases, liquid-phase diffusion transport, and key reactions within the liquid phase. In “Application Systems of Gas–Liquid Interface Transport of Air Plasma-Generated RONS for Plant Disease Control” section, we provided interpretations of measurable bulk liquid RONS for RONS transport across phases, alongside insights gained from our previous studies, using a laboratory-built air-plasma device developed for plant pathogen control. In addition, the selective synthesis of N2O5gas, which can supply short-lived intermediates near the gas–liquid interface, was introduced based on a quantitative analysis of air plasma effluent gas chemistry. In “Future Prospects on Gas–Liquid Interfacial Air Plasmas” section, future challenges regarding complicated interactions at the plasma–liquid interface were discussed. The importance of developing a fundamental basis for plasma–liquid interactions by reducing the degree of freedom in liquid motion is emphasized to untangle the complexity of the interactions associated with GLIPs.
Plasma technology, in the controlled supply of various reactive species and their mixtures at desired concentrations to a target, plays a major role in processing plasma, notably in semiconductor processes. The establishment of biphasic RONS transport in GLIPs using liquid water can substantially contribute to the advancement of research in diverse fields utilizing GLIPs. These fields span medicine, agriculture, and green chemistry and hold immense potential for fostering innovative applications in the foreseeable future.
Data Availability
Data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- APP:
-
Atmospheric pressure plasma
- GLIP:
-
Gas–liquid interfacial plasma
- ROS:
-
Reactive oxygen species
- RNS:
-
Reactive nitrogen species
- RONS:
-
Reactive oxygen and nitrogen species
- DBD:
-
Dielectric barrier discharge
References
Fridman A, Chirokov A, Gutsol A (2005) Non-thermal atmospheric pressure discharges. J Phys D Appl Phys 38:R1. https://doi.org/10.1088/0022-3727/38/2/R01
Bruggeman PJ, Kushner MJ, Locke BR et al (2016) Plasma–liquid interactions: a review and roadmap. Plasma Sources Sci Technol 25:053002. https://doi.org/10.1088/0963-0252/25/5/053002
Adamovich I, Agarwal S, Ahedo E et al (2022) The 2022 plasma roadmap: low temperature plasma science and technology. J Phys D Appl Phys 55:373001. https://doi.org/10.1088/1361-6463/ac5e1c
Fridman G, Friedman G, Gutsol A et al (2008) Applied plasma medicine. Plasma Process Polym 5:503–533. https://doi.org/10.1002/ppap.200700154
Kong MG, Kroesen G, Morfill G et al (2009) Plasma medicine: an introductory review. New J Phys 11:115012. https://doi.org/10.1088/1367-2630/11/11/115012
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D Appl Phys 45:263001. https://doi.org/10.1088/0022-3727/45/26/263001
Puač N, Gherardi M, Shiratani M (2018) Plasma agriculture: a rapidly emerging field. Plasma Process Polym 15:1700174. https://doi.org/10.1002/ppap.201700174
Gao Y, Francis K, Zhang X (2022) Review on formation of cold plasma activated water (PAW) and the applications in food and agriculture. Food Res Int 157:111246. https://doi.org/10.1016/j.foodres.2022.111246
Li S, Medrano AJ, Hessel V, Gallucci F (2018) Recent progress of plasma-assisted nitrogen fixation research: a review. Processes 6:248. https://doi.org/10.1007/s00705-018-3909-4
Bogaerts A, Tu X, Whitehead JC et al (2020) The 2020 plasma catalysis roadmap. J Phys D Appl Phys 53:443001. https://doi.org/10.1088/1361-6463/ab9048
Kumagai S, Nishigori C, Takeuchi T et al (2021) Towards prevention and prediction of infectious diseases with virus sterilization using ultraviolet light and low-temperature plasma and bio-sensing devices for health and hygiene care. Jpn J Appl Phys 61:SA0808. https://doi.org/10.35848/1347-4065/ac1c3d
Kaneko T, Kato H, Yamada H et al (2021) Functional nitrogen science based on plasma processing: quantum devices, photocatalysts and activation of plant defense and immune systems. Jpn J Appl Phys 61:SA0805. https://doi.org/10.35848/1347-4065/ac25dc
Šerá B, Šerý M (2018) Non-thermal plasma treatment as a new biotechnology in relation to seeds, dry fruits, and grains. Plasma Sci Technol 20:44012. https://doi.org/10.1088/2058-6272/aaacc6
Kaushik NK, Ghimire B, Li Y et al (2018) Biological and medical applications of plasma-activated media, water and solutions. Biol Chem 400:39–62. https://doi.org/10.1515/hsz-2018-0226
Thirumdas R, Kothakota A, Annapure U et al (2018) Plasma activated water (PAW): chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci Technol 77:21–31. https://doi.org/10.1016/j.tifs.2018.05.007
Zhou R, Zhou R, Prasad K et al (2018) Cold atmospheric plasma activated water as a prospective disinfectant: the crucial role of peroxynitrite. Green Chem 20:5276–5284. https://doi.org/10.1039/C8GC02800A
Hori Y, Iwata N, Gamaleev V et al (2021) Identification of key neutral species in atmospheric-pressure plasma for promoting proliferation of fibroblast cells. Plasma Process Polym 18:2000225. https://doi.org/10.1002/ppap.202000225
Lukes P, Dolezalova E, Sisrova I, Clupek M (2014) Aqueous-phase chemistry and bactericidal effects from an air discharge plasma in contact with water: evidence for the formation of peroxynitrite through a pseudo-second-order post-discharge reaction of H2O2 and HNO2. Plasma Sources Sci Technol 23:015019. https://doi.org/10.1088/0963-0252/23/1/015019
Ikawa S, Tani A, Nakashima Y, Kitano K (2016) Physicochemical properties of bactericidal plasma-treated water. J Phys D Appl Phys 49:425401. https://doi.org/10.1088/0022-3727/49/42/425401
Kondeti VSSK, Phan CQ, Wende K et al (2018) Long-lived and short-lived reactive species produced by a cold atmospheric pressure plasma jet for the inactivation of Pseudomonas aeruginosa and Staphylococcus aureus. Free Radic Biol Med 124:275–287. https://doi.org/10.1016/j.freeradbiomed.2018.05.083
Aboubakr HA, Gangal U, Youssef MM et al (2016) Inactivation of virus in solution by cold atmospheric pressure plasma: identification of chemical inactivation pathways. J Phys D Appl Phys 49:204001. https://doi.org/10.1088/0022-3727/49/20/204001
Nayak G, Andrews AJ, Marabella I et al (2020) Rapid inactivation of airborne porcine reproductive and respiratory syndrome virus using an atmospheric pressure air plasma. Plasma Process Polym 17:1900269. https://doi.org/10.1002/ppap.201900269
Shimada K, Takashima K, Kimura Y et al (2020) Humidification effect of air plasma effluent gas on suppressing conidium germination of a plant pathogenic fungus in the liquid phase. Plasma Process Polym 17:e1900004. https://doi.org/10.1002/ppap.201900004
Takashima K, Hu Y, Goto T et al (2020) Liquid spray transport of air-plasma-generated reactive species toward plant disease management. J Phys D Appl Phys 53:354004. https://doi.org/10.1088/1361-6463/ab87bd
Hanbal SE, Takashima K, Miyashita S et al (2018) Atmospheric-pressure plasma irradiation can disrupt tobacco mosaic virus particles and RNAs to inactivate their infectivity. Arch Virol 163:2835–2840. https://doi.org/10.1007/s00705-018-3909-4
Sasaki S, Osana S, Kubota T et al (2022) Human coronavirus inactivation by atmospheric pressure helium plasma. J Phys D Appl Phys 55:295203. https://doi.org/10.1088/1361-6463/ac6a8c
Sasaki S, Kanzaki M, Kaneko T (2016) Calcium influx through TRP channels induced by short-lived reactive species in plasma-irradiated solution. Sci Rep 6:25728. https://doi.org/10.1038/srep25728
Sasaki S, Honda R, Hokari Y et al (2016) Characterization of plasma-induced cell membrane permeabilization: focus on OH radical distribution. J Phys D Appl Phys 49:334002. https://doi.org/10.1088/0022-3727/49/33/334002
Kaneko T, Sasaki S, Takashima K, Kanzaki M (2017) Gas–liquid interfacial plasmas producing reactive species for cell membrane permeabilization. J Clin Biochem Nutr 60:3–11. https://doi.org/10.3164/jcbn.16-73
Sasaki S, Hokari Y, Kumada A et al (2018) Direct plasma stimuli including electrostimulation and OH radical induce transient increase in intracellular Ca2+ and uptake of a middle-size membrane-impermeable molecule. Plasma Process Polym 15:e1700077. https://doi.org/10.1002/ppap.201700077
Honda R, Sasaki S, Takashima K et al (2020) Characterization of middle-molecule introduction into cells using mm-scale discharge in saline. Jpn J Appl Phys 59:040904. https://doi.org/10.35848/1347-4065/ab7d7e
Kawase M, Chen W, Kawaguchi K et al (2020) TRPA1 and TRPV1 channels participate in atmospheric-pressure plasma-induced [Ca2+]i response. Sci Rep 10:9687. https://doi.org/10.1038/s41598-020-66510-y
Sasaki S, Zheng Y, Mokudai T et al (2020) Continuous release of O2−/ONOO− in plasma-exposed HEPES-buffered saline promotes TRP channel-mediated uptake of a large cation. Plasma Process Polym 17:e1900257. https://doi.org/10.1002/ppap.201900257
Ochi A, Konishi H, Ando S et al (2016) Management of bakanae and bacterial seedling blight diseases in nurseries by irradiating rice seeds with atmospheric plasma. Plant Pathol 66:67–76. https://doi.org/10.1111/ppa.12555
Tsukidate D, Takashima K, Sasaki S et al (2022) Activation of plant immunity by exposure to dinitrogen pentoxide gas generated from air using plasma technology. PLoS ONE 17:e0269863. https://doi.org/10.1371/journal.pone.0269863
Yokoyama T, Miyazaki S, Akagi H et al (2021) Kinetics of bacterial inactivation by peroxynitric acid in the presence of organic contaminants. Appl Environ Microbiol 87:e01860-e1920. https://doi.org/10.1007/s00705-018-3909-4
Tian W, Kushner MJ (2014) Atmospheric pressure dielectric barrier discharges interacting with liquid covered tissue. J Phys D Appl Phys 47:165201. https://doi.org/10.1088/0022-3727/47/16/165201
Tian W, Kushner MJ (2015) Long-term effects of multiply pulsed dielectric barrier discharges in air on thin water layers over tissue: stationary and random streamers. J Phys D Appl Phys 48:494002. https://doi.org/10.1088/0022-3727/48/49/494002
Lucas JA (2009) Plant pathology and plant pathogens. Wiley, Hoboken
Kimura Y, Takashima K, Sasaki S, Kaneko T (2019) Investigation on dinitrogen pentoxide roles on air plasma effluent exposure to liquid water solution. J Phys D Appl Phys 52:064003. https://doi.org/10.1088/1361-6463/aaf15a
Takashima K, Bin Ahmad Nor AS, Ando S et al (2020) Evaluation of plant stress due to plasma-generated reactive oxygen and nitrogen species using electrolyte leakage. Jpn J Appl Phys 60:010504. https://doi.org/10.35848/1347-4065/abcf5b
Takashima K, Kaneko T (2017) Ozone and dinitrogen monoxide production in atmospheric pressure air dielectric barrier discharge plasma effluent generated by nanosecond pulse superimposed alternating current voltage. Plasma Sources Sci Technol 26:065018. https://doi.org/10.1088/1361-6595/aa7082
Sasaki S, Takashima K, Kaneko T (2021) Portable plasma device for electric N2O5 production from air. Ind Eng Chem Res 60:798–801. https://doi.org/10.1021/acs.iecr.0c04915
Anifowose AJ, Takeda K, Sakugawa H (2015) Novel fluorometric method for the determination of production rate and steady-state concentration of photochemically generated superoxide radical in seawater using 3′,6′-(diphenylphosphinyl)fluorescein. Anal Chem 87:11998–12005. https://doi.org/10.1021/acs.analchem.5b00917
Lankone RS, Deline AR, Barclay M, Fairbrother DH (2020) UV–Vis quantification of hydroxyl radical concentration and dose using principal component analysis. Talanta 218:121148. https://doi.org/10.1016/j.talanta.2020.121148
Yonemori S, Ono R (2014) Flux of OH and O radicals onto a surface by an atmospheric-pressure helium plasma jet measured by laser-induced fluorescence. J Phys D Appl Phys 47:125401. https://doi.org/10.1088/0022-3727/47/12/125401
Ono R (2016) Optical diagnostics of reactive species in atmospheric-pressure nonthermal plasma. J Phys D Appl Phys 49:083001. https://doi.org/10.1088/0022-3727/49/8/083001
Kolb CE, Cox RA, Abbatt JPD et al (2010) An overview of current issues in the uptake of atmospheric trace gases by aerosols and clouds. Atmos Chem Phys 10:10561–10605. https://doi.org/10.5194/acp-10-10561-2010
Oinuma G, Nayak G, Du Y, Bruggeman PJ (2020) Controlled plasma–droplet interactions: a quantitative study of OH transfer in plasma–liquid interaction. Plasma Sources Sci Technol 29:95002. https://doi.org/10.1088/1361-6595/aba988
Nayak G, Oinuma G, Yue Y et al (2021) Plasma-droplet interaction study to assess transport limitations and the role of ⋅OH, O⋅, H⋅, O2 (a1Δg), O3, He(23S) and Ar(1s5) in formate decomposition. Plasma Sources Sci Technol 30:115003. https://doi.org/10.1088/1361-6595/ac2676
Nayak G, Wang J, Li R et al (2023) Non-OH-driven liquid-phase chemistry in water microdroplets. Plasma Process Polym. https://doi.org/10.1002/ppap.202200222
Lieberman MA, Lichtenberg AJ (2005) Principles of plasma discharges and materials processing. Wiley, Hoboken
JohnáElliot A (1990) Estimation of rate constants for near-diffusion-controlled reactions in water at high temperatures. J Chem Soc Faraday Trans 86:1539–1547. https://doi.org/10.1039/FT9908601539
Hervé du Penhoat M-A, Goulet T, Frongillo Y et al (2000) Radiolysis of liquid water at temperatures up to 300 °C: a Monte Carlo simulation study. J Phys Chem A 104:11757–11770. https://doi.org/10.1021/jp001662d
Chameides WL (1984) The photochemistry of a remote marine stratiform cloud. J Geophys Res Atmos 89:4739–4755. https://doi.org/10.1029/JD089iD03p04739
Holz M, Heil SR, Sacco A (2000) Temperature-dependent self-diffusion coefficients of water and six selected molecular liquids for calibration in accurate 1H NMR PFG measurements. Phys Chem Chem Phys 2:4740–4742. https://doi.org/10.1039/B005319H
Johnson PN, Davis RA (1996) Diffusivity of ozone in water. J Chem Eng Data 41:1485–1487. https://doi.org/10.1021/je9602125
Roth JA, Sullivan DE (1981) Solubility of ozone in water. Ind Eng Chem Fundam 20:137–140. https://doi.org/10.1021/i100002a004
Novoselov AG, Chebotar AV, Sorokin SA et al (2022) Oxygen diffusion in water. Estimation of the coefficients of molecular diffusion. AIP Conf Proc 2486:20016. https://doi.org/10.1063/5.0106867
Zacharia IG, Deen WM (2005) Diffusivity and solubility of nitric oxide in water and saline. Ann Biomed Eng 33:214–222. https://doi.org/10.1007/s10439-005-8980-9
Kramers H, Blind MPP, Snoeck E (1961) D2. Absorption of nitrogen tetroxide by water jets. Chem Eng Sci 14:115–123. https://doi.org/10.1016/0009-2509(61)85062-8
Sander R (2015) Compilation of Henry’s law constants (version 4.0) for water as solvent. Atmos Chem Phys 15:4399–4981. https://doi.org/10.5194/acp-15-4399-2015
White FM (2006) Viscous fluid flow, 3rd edn. McGraw-Hill New York, Singapore
Løgager T, Sehested K (1993) Formation and decay of peroxynitrous acid: a pulse radiolysis study. J Phys Chem 97:6664–6669. https://doi.org/10.1021/j100333a025
Bielski BHJ, Cabelli DE, Arudi RL, Ross AB (1985) Reactivity of HO2/O−2 radicals in aqueous solution. J Phys Chem Ref Data 14:1041–1100. https://doi.org/10.1063/1.555739
Goldstein S, Czapski G (1995) The reaction of NO· with O2·− and HO2·−: a pulse radiolysis study. Free Radic Biol Med 19:505–510. https://doi.org/10.1016/0891-5849(95)00034-U
Huie RE, Padmaja S (1993) The reaction of NO with superoxide. Free Radic Res Commun 18:195–199. https://doi.org/10.3109/10715769309145868
Goldstein S, Lind J, Merényi G (2005) Chemistry of peroxynitrites as compared to peroxynitrates. Chem Rev 105:2457–2470. https://doi.org/10.1021/cr0307087
Gardoni D, Vailati A, Canziani R (2012) Decay of ozone in water: a review. Ozone Sci Eng 34:233–242. https://doi.org/10.1080/01919512.2012.686354
Hoigné J, Bader H, Haag WR, Staehelin J (1985) Rate constants of reactions of ozone with organic and inorganic compounds in water—III. Inorganic compounds and radicals. Water Res 19:993–1004. https://doi.org/10.1016/0043-1354(85)90368-9
Park JY, Lee YN (1988) Solubility and decomposition kinetics of nitrous acid in aqueous solution. J Phys Chem 92:6294–6302. https://doi.org/10.1021/j100333a025
Goldstein S, Czapski G (1995) Kinetics of nitric oxide autoxidation in aqueous solution in the absence and presence of various reductants. The nature of the oxidizing intermediates. J Am Chem Soc 117:12078–12084. https://doi.org/10.1021/ja00154a007
Grätzel M, Henglein A, Lilie J, Beck G (1969) Pulsradiolytische untersuchung einiger elementarprozesse der oxydation und reduktion des nitritions. Ber Bunsenges Phys Chem 73:646–653. https://doi.org/10.1002/bbpc.19690730707
Løgager T, Sehested K (1993) Formation and decay of peroxynitric acid: a pulse radiolysis study. J Phys Chem 97:10047–10052. https://doi.org/10.1021/j100141a025
Régimbal J-M, Mozurkewich M (1997) Peroxynitric acid decay mechanisms and kinetics at low pH. J Phys Chem A 101:8822–8829. https://doi.org/10.1021/jp971908n
Bertram TH, Thornton JA (2009) Toward a general parameterization of N2O5 reactivity on aqueous particles: the competing effects of particle liquid water, nitrate and chloride. Atmos Chem Phys 9:8351–8363. https://doi.org/10.5194/acp-9-8351-2009
Noble RW, Gibson QH (1970) The reaction of ferrous horseradish peroxidase with hydrogen peroxide. J Biol Chem 245:2409–2413. https://doi.org/10.1016/S0021-9258(18)63167-9
Mack J, Bolton JR (1999) Photochemistry of nitrite and nitrate in aqueous solution: a review. J Photochem Photobiol A Chem 128:1–13. https://doi.org/10.1016/S1010-6030(99)00155-0
Griess P (1879) Bemerkungen zu der abhandlung der HH. Weselsky und Benedikt “Ueber einige Azoverbindungen.” Ber Dtsch Chem Gesellschaft 12:426–428. https://doi.org/10.1002/cber.187901201117
Trinder P (1969) Determination of blood glucose using an oxidase-peroxidase system with a non-carcinogenic chromogen. J Clin Pathol 22:158–161. https://doi.org/10.1136/jcp.22.2.158
Galib M, Limmer DT (2021) Reactive uptake of N2O5 by atmospheric aerosol is dominated by interfacial processes. Science 371:921–925. https://doi.org/10.1126/science.abd7716
Golkowski M, Golkowski C, Leszczynski J et al (2012) Hydrogen-peroxide-enhanced nonthermal plasma effluent for biomedical applications. IEEE Trans Plasma Sci 40:1984–1991. https://doi.org/10.1109/TPS.2012.2200910
Eisenberg G (1943) Colorimetric determination of hydrogen peroxide. Ind Eng Chem Anal Ed 15:327–328. https://doi.org/10.1021/i560117a011
Lewis D (1958) The absorption spectrum of the Titanium(IV)–hydrogen peroxide complex. J Phys Chem 62:1145–1146. https://doi.org/10.1021/j150567a036
Koppenol WH, Moreno JJ, Pryor WA et al (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chem Res Toxicol 5:834–842. https://doi.org/10.1021/tx00030a017
Kissner R, Nauser T, Bugnon P et al (1997) Formation and properties of peroxynitrite as studied by laser flash photolysis, high-pressure stopped-flow technique, and pulse radiolysis. Chem Res Toxicol 10:1285–1292. https://doi.org/10.1021/tx970160x
Fridman A (2008) Plasma chemistry. Cambridge University Press, Cambridge
Haines LB, Adkins H (1925) The reaction of certain organic compounds with nitrogen pentoxide. J Am Chem Soc 47:1419–1426. https://doi.org/10.1021/ja01682a029
Atkinson R, Baulch DL, Cox RA et al (2004) Evaluated kinetic and photochemical data for atmospheric chemistry: volume I-gas phase reactions of Ox, HOx, NOx and SOx species. Atmos Chem Phys 4:1461–1738. https://doi.org/10.5194/acp-4-1461-2004
Tian W, Lietz AM, Kushner MJ (2016) The consequences of air flow on the distribution of aqueous species during dielectric barrier discharge treatment of thin water layers. Plasma Sources Sci Technol 25:55020. https://doi.org/10.1088/0963-0252/25/5/055020
Takeda K, Sasaki S, Luo W et al (2021) Experimental detection of liquid-phase OH radical decay originating from atmospheric-pressure plasma exposure. Appl Phys Express 14:056001. https://doi.org/10.35848/1882-0786/abf80e
Foster JE, Mujovic S, Groele J, Blankson IM (2018) Towards high throughput plasma based water purifiers: design considerations and the pathway towards practical application. J Phys D Appl Phys 51:293001. https://doi.org/10.1088/1361-6463/aac816
Dickenson A, Walsh JL, Hasan MI (2021) Electromechanical coupling mechanisms at a plasma–liquid interface. J Appl Phys 129:213301. https://doi.org/10.1063/5.0045088
Park S, Choe W, Lee H et al (2021) Stabilization of liquid instabilities with ionized gas jets. Nature 592:49–53. https://doi.org/10.1038/s41586-021-03359-9
Korovin VM (2017) Effect of tilted electrostatic field on the Kelvin–Helmholtz instability in a liquid dielectric and gas flow. Tech Phys 62:1316–1321. https://doi.org/10.1134/S1063784217090134
Berendsen CWJ, van Veldhuizen EM, Kroesen GMW, Darhuber AA (2015) Marangoni flows induced by atmospheric-pressure plasma jets. J Phys D Appl Phys 48:025203. https://doi.org/10.1088/0022-3727/48/2/025203
Shirai N, Kaneko T, Takamura Y, Sasaki K (2022) Effect of atmospheric-pressure plasma irradiation on the surface tension of water. J Phys D Appl Phys 55:15LT01. https://doi.org/10.1088/1361-6463/ac48ad
Kunishima Y, Takashima K, Kaneko T (2019) Apparent reduced electric field control with nanosecond pulse width in a DC discharge for nitrogen vibrational excitation. Jpn J Appl Phys 58:060908. https://doi.org/10.7567/1347-4065/ab1e58
Acknowledgements
The authors thank Hideki Takahashi, Atsushi Higashitani, Nobuyuki Uozumi, Sugihiro Ando, Yasuhiro Ishimaru, Makoto Kanzaki, Hiroyasu Kanetaka, Kazuki Takeda, Shouki Takeshi, Hiroto Iwamoto, Yuki Kunishima, Takafumi Sekine, Ahmad Shahir bin Ahmad Nor, Tsubasa Goto, Yue Hu, Wencheng Luo, Kenji Nihei, Yutaka Kimura, Keisuke Shimada, Hideaki Konishi, and Kento Kikuta for their support and collaboration. Also, the authors would like to thank Editage (www.editage.jp) for English language editing.
Funding
This work was supported by JSPS KAKENHI (Grant Nos. 26600124, 16K13708, 17H04817, 18H03687, 20H01890, 21K13906, 22J21877, and 22H04947), FRiD Tohoku University (Project 1904), Plasma-bio Consortium (Grant Nos. 01222001 and 01223002), cLPS Nagoya University, and RIEC Tohoku University.
Author information
Authors and Affiliations
Contributions
Toshiro Kaneko was involved in conceptualization, writing-review, supervision, project management, and funding acquisition. Keisuke Takashima was involved in conceptualization, investigation, writing, visualization, and funding acquisition. Shota Sasaki was involved in conceptualization, investigation, writing, editing, visualization, and funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kaneko, T., Takashima, K. & Sasaki, S. Integrated Transport Model for Controlled Delivery of Short-Lived Reactive Species via Plasma-Activated Liquid with Practical Applications in Plant Disease Control. Plasma Chem Plasma Process 44, 1165–1201 (2024). https://doi.org/10.1007/s11090-024-10461-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-024-10461-z