Abstract
Rice seeds (Oryza sativa L.) have been treated with cold atmospheric plasma in air both directly in a dielectric barrier discharge and indirectly by gliding arc discharge with plasma activated mist. Comparisons of impacts of the two methods on rice seeds germination and physiological parameters are presented.
Plasma has been found to increase seeds hydrophilization and water uptake through decrease of liquid contact angle and increase of total surface free energy of seed’s coat. Germination of treated seeds and their seedling growth parameters are enhanced by plasma application. After 20 min of seeds direct and indirect exposures respectively, germination potential increases by 36.73 and 50.4%, germination rate by 26.0 and 30.0%, and germination index by 25.92 and 36.53%. Also, total shoot length increases by 42.2 and 48.5%, and total root length by 15.93 and 22.42%. Plasma enhances physiological changes by increasing nonenzymatic antioxidants substances which in turn increase the tolerance against abiotic stresses. After 20 min for direct and indirect exposures respectively, free proline increases by 30.0 and 40.0%, total soluble carbohydrates by 49.7 and 54.6%. Also, cellular reactive oxygen species (ROS) increase by 52.0 and 60.0%, and malondialdehyde (MDA) decreases by 68.0 and 88.0%. Indirect exposure of rice seeds by plasma activated mist shows enhanced effects on germination and physiological parameters compared to direct one and could be more practical when applied to large scale seeds plasma treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Facing the rise in food demand caused by increasing world population, one should use innovative techniques to increase crops productivity and combat global warming effects which imposes severe conditions on major crops in many countries [1, 2]. Crop harvest can be subjected to environmental abiotic stresses caused by water deficit, excess elevated temperature, UV radiation, water salinity, and soil heavy metal loading, which can reduce crop germination and limit productivity [3]. Fortunately, plants in normal conditions have their own defense mechanisms that permit them to initiate several molecular, cellular, and physiological modifications to react against all those abiotic stress agents and protect themselves from their oxidative damaging effects [4]. Under abiotic stress conditions, plant cells stimulate intracellular production of reactive oxygen species (ROS) which include free radicals like hydroxyl OH•, and superoxide anions O2•− as well as non-radicals like hydrogen peroxide H2O2, and singlet oxygen 1O2 [5]. ROS at high concentration in plant cells are toxic through oxidative damage which may cause cell death. However, at lower concentrations ROS act as signaling molecules in the control of various cellular processes to maintain plant cells normal development and improve their tolerance to stress [6]. Abiotic stresses can also trigger the accumulation of compatible solutes such as proline and soluble sugar level, which take part in osmotic protection [7]. Proline is a type of amino acid which constitutes the main building blocks of protein [8]. It acts as a signaling molecule to help cells to achieve osmotic pressure adjustment and regulate complex metabolic and developmental processes, this may reduce cell degradation due to stress-induced oxidative damage. Soluble sugars contribute also to the plant defense mechanism against environmental stress by regulating osmotic adjustment, protecting membranes, and scavenging toxic reactive oxygen species [9]. High levels of ROS can also inflict direct damage to lipids causing lipid peroxidation. The contents of malondialdehyde (MDA) are the end-product of lipid peroxidation and reflect the degree of damage due to adverse conditions [10]. For severe abiotic conditions one should introduce a special treatment that may help plants to withstand stress. Nonthermal plasma (NTP), particularly cold atmospheric plasma (CAP) has been proposed to treat seeds for increase crops productivity, fight pathogens and prepare plants to develop their own defense mechanisms against severe conditions which they may be subjected during their growth [11]. Plasma is mainly composed of charged particles, electric fields, reactive species such as oxygen and nitrogen species (ROS and RNS), Ozone (O3) and UV radiation. All interact with plant biological systems and can affect their different metabolites [12]. Rice (Oryza sativa L.) is one of the most important nutritional ingredients, it serves as the staple food throughout much of the world [13]. Plasma has been previously applied to treat rice seeds; many works have proven the effectiveness of plasma treatment in enhancing rice productivity as well as protecting seedlings from abiotic severe conditions. Tan et al. [14], used arc-tooth-shaped corona discharge field to treat rice seeds. They examined physiological and biochemical indexes and have found that plasma increased rice seed vigor by promoting membrane repair and improving metabolic and anti-oxidation enzymes activities. Teerakawanich et al. [15], investigated two separate configurations based on multipoint-to-plane electrodes under ambient atmosphere in open air and have found that improvement in the wetting properties has been dependent on the mutual relationships between the apparent contact angle and water imbibition. Penado et al. [16], explored the effect of atmospheric air plasma jet treatment on the seed germination of rice and have found that plasma treatment of rice seeds significantly affected germ growth but not germination count which have been attributed to change of seeds surface roughness. Amnuaysin et al. [17], investigated the effect of plasma generated by an atmospheric air dielectric barrier discharge (DBD) on rice seed germination and early growth and they have pointed that plasma had the potential to promote seed germination and seedling growth by changing the surfaces of rice seeds. Sheteiwy et al. [18], studied the effects of cold plasma treatment and salicylic acid (SA) and their combination on the physiological parameters and metabolism of two cultivars of Oryza sativa. They have deduced that SA priming and cold plasma treatment either alone or combined improved plant uptake of nutrients in both cultivars under stress conditions and enhance salinity tolerance via decreasing the oxidative damage of plant membranes by promoting both enzymatic and non-enzymatic antioxidant activities in the rice seedling grown under higher salinity level. Hashizume et al. [19], examined the effects of plasma irradiation and treatment with plasma‐activated Ringer's lactate solution (PAL) on rice seedlings in a paddy field. They have deduced that in the PAL treatment, the traits related to the growth of the main stem were improved, whereas the grain yield of the whole plant was decreased due to the suppression of tillering shoot growth. Tanakaran et al. [20], studied the influence of atmospheric non-thermal plasma generated by a multi-pin plasma generator on the enhancement of early growth jasmine rice seeds. Their results confirmed that the application of plasma has improved not only the germination and the growth of jasmine rice seeds but also the protein content of pre-germinated brown jasmine rice seeds. Rashid et al. [21], investigated the combined effects of low pressure (100 torr) glow air discharge on paddy seeds and plasma activated waters applied as foliar spray to the plants grown from the treated seeds on plant growth, yield and total soluble protein and sugar concentrations in the produced paddy grains. They have found that these combined effects enhance paddy seed germination rate, plants growth parameters, and defense mechanisms of plants. Rongsangchaicharean et al. [22] used two large-scale CAP, streamer corona plasma (SCP) and dielectric barrier discharge (DBD) plasma, to enhance rice seed vigor through surface modification and functionalization and have concluded that both have improved seed vigor, water uptake and permeability than non-treated seeds and initiate better germination parameters and fast radicle emergence.
In this study we will use two types of plasma discharges to treat rice seeds (Oryza sativa L.). First, a dielectric barrier discharge (DBD) applied to rice seeds as a direct method in air. Second, a gliding arc discharge (GAD) applied as indirect method through plasma activated mist (PAMi) which we will abbreviate as GAD-PAMi. We will study their effects on germination parameters as germination potential, germination rate, and germination index, as well as seedling growth as total shoot and root lengths and vigor index, also physiological parameters as free proline, soluble carbohydrate, cellular ROS, and MDA.
Materials and Methods
Experimental Set Up
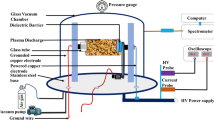
For direct treatment method, the rice seeds are directly placed in plasma between the electrodes of a DBD. The DBD arrangement is shown in Fig. 1. The atmospheric discharge occurs between two stainless steel electrodes of 50 mm diameter and 2 mm thickness each with inter-electrodes distance of 6 mm in which we inserted a Pyrex glass plate of 2 mm thickness as a dielectric placed on the lower electrode and carrying on it the seed specimens within a 4 mm air gap. The upper electrode is biased with 50 Hz AC high voltage supplied by a 9 kV step-up transformer, rating 20 mA maximum with its primary windings connected to a Variac attached to the upper electrode while the lower one is grounded. The applied high voltage is measured with a 1000:1 high voltage probe (Tektronix P6015A, 75 MHz) and the current with a Rogowski coil (Pearson 4100, 1 V/1A). The voltage-drop across a 100 nF capacitor is measured using a 10:1 oscilloscope probe. Measured signals are displayed on a four-channel digital oscilloscope (Tektronix TDS2024C, 200 MHz). Air delivered by an air compressor is introduced by a mass flow controller (MFC) (Omega FMA-A2409) and is adjusted to flow rate of 50 SLM.
The indirect method is applied through a gliding arc discharge in which flows a plasma activated mist (GAD-PAMi) [23]. The setup arrangement is shown in Fig. 2.
Rice seeds are subjected to PAMi formed by water droplets of 30 μm diameter produced by an Ultrasonic nebulizer operating in the range from 0.8 to 1.65 MHz. The GAD consists of two knife-shaped stainless-steel electrodes of 80 mm long, 20 mm wide on its largest position and the gap at the electrodes' neck is adjusted to around 2 mm. An Ac power source supplying up to 9 kV and 30 mA maximum. The working gas is compressed air supplied by a compressor with volume flow rate of 16 SLM fed through a nozzle positioned between the two electrodes. The spacing between the electrodes' extreme tips and the seeds placed in an acrylic box is around 12 cm.
Seed Coat Temperature Measurement
Infrared images of seeds coats are measured directly after plasma treatment using a thermal imager sensor FLIR A5sc (Oregon, USA) with spatial resolution of 2.78 mrad and spectral resolution of 7.5–13 μm. The IR sensor is set 20 cm from specimen. focused on an area of 0.12 cm2 on the middle of the treated seed of 0.1 cm2. The sensor is factory calibrated, and the values of the measuring conditions are fed to the original software program as follows: ambient temperature 26.6 °C, relative humidity 60%. The IR emissivity e for the seed’s coat material is taken to be e = 0.97.
Monitoring of Water Mist Parameters
Water mist passing through the gliding arc has been measured after condensation directly next to seeds plasma exposure. Parameters of plasma treated water are measured by Edge 2000, HI2020-02, from Hanna Instruments (USA) equipped with corresponding sensors. For pH value we use HI11310 electrode, for electric conductivity (EC) we use HI763100 Electrode and for oxidation reduction potential (ORP) we use HI 98120. Hydrogen peroxide (H2O2) concentration in treated water is quantified employing test strips from Macherey–Nagel Quatofix peroxide (Germany). Range 1–100 mg/L H2O2, Z101680. Nitrate (NO3−) in the range of 10–500 mg/L, Z 166413–100 EA and Nitrite (NO2−) in the range of 1–80 mg/L, Z166421–100EA are measured by Quatofix test strips from Sigma-Aldrich.
Optical Emission Spectroscopy (OES)
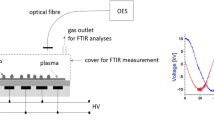
Optical emission spectroscopy on the UV–vis range has been used to identify some atomic and molecular species produced during the discharge. Measurements are done using a fiber optics spectrometer AVANTES AvaSpec-ULS2048 StarLine with a grating of 2400 grooves/mm at the wavelength range 200–1100 nm and resolution 0.9 nm. A collimating quartz lens was employed to focus the light beam from the discharge in between the electrodes.
Scanning Electron Microscopy
Scanning electron microscopy (SEM) carried out by JEOL JSM-6510 lv Japan is used for seed coat visualization for untreated and plasma treated seeds with DBD at different time intervals.
FTIR Surface Analysis of Seed’s Surface
Infrared analysis using attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy is a fast technique which allows the identification of chemical changes occurring on the specimen surface due to plasma treatment. The measurements were performed using an FTIR spectrometer Vertex 70 (Bruker-Germany) equipped with vertical ATR device which is a surface sensitive method due to its inherent low depth of penetration. The spectrometer is continuously purged with nitrogen gas. The internal reflection element is a diamond crystal. A total of 32 scans per analysis were used with a resolution of 4 cm−1 are taken for spectrum integration in the wavenumber range from 4000 to 400 cm−1. ATR-FTIR analysis has been carried out on the untreated and plasma treated seeds’ specimens.
Wettability, Contact Angle and Surface Energy of Seed’s Coating Surface
Wettability of rice seed’s coat is estimated by measuring the contact angle of liquid drop on the seed’s surface. The contact angle is defined by the angle between the solid surface and the tangent to the surface of the drop at the point of contact [24]. We use distilled water and glycerol as droplets on the surfaces of the seeds before "control" and after plasma treatment for different time periods, then, we measured a contact angle by using the sessile drop technique from images of water droplets onto seed surfaces. The contact angle θ is given by Young's equation [25]:
where γsv is the surface free energy of solid substrate touching vapor, γlv is the surface free energy of liquid touching vapor, and γsl is the solid–liquid interfacial free energy. The surface energy is calculated using the two liquids method using distilled water and glycerol by Fowke’s approximation [26].
where θ is the testing liquid’s contact angle, \(\gamma_{l}\) is the liquid surface tension having respective polar and dispersive components \(\gamma_{l}^{p}\) and \(\gamma_{l}^{d}\). The polar and dispersive components of solid-surface tension \( \gamma_{s} \) are given by:
For two liquids i and j,
By using the values of the surface tension and its polar and dispersive components of the test liquids, components of the surface energy of the solid \(\gamma_{s}^{p}\) and \(\gamma_{s}^{d}\) can be determined from Eqs. 4 and 5, and the sum of the two quantities represents the total energy of seed coat. The polar and dispersive components values of testing liquids are given in Table 1.
Determination of Seeds Water Uptake
For spontaneous imbibition determination, 100 rice seeds have been weighed on a digital scales (SHIMADZU LIBROR EB-430HU, Japan) of accuracy 1 mg directly after plasma treatment to determined their dry weight (DW). Then, seeds have been hydrated with distilled water and covered to prevent evaporation, this is repeated every 2 h for twelve hours at room temperature. Hydrated seeds have been dried and weighed to determine their fresh weight (FW). Water content (WC) per seed is calculated using:
Calculation of Seeds Germination
In this study we use an Egyptian rice (Oryza sativa L.) variety (cv. Sakha 104, Agriculture Research Institute, Giza, Egypt), popularly cultivated in the Nile Delta. Rice seeds were obtained from a local farm in Al Sharqia governorate coming from a forgoing harvest on mid-September 2022, carefully stored in a dry location at ultimate temperature of 23 °C and relative humidity (RH) < 60%. We selected for this investigation healthy ripe seeds of regular size without apparent defects. A total number of 156 rice seeds are treated by plasma for each treatment time period for 2, 5, 10, 15 and 20 min, and then soaked in water for 10 h to accelerate germination, disinfected it with alcohol (70%) for one minute, then rinse with distilled water and prepare for germination. For statistical purposes, 156 plasma-treated seeds were distributed, during each period, into three groups of 52 seeds arranged over two layers of filter paper in three Petri dishes (9 cm in diameter). Each dish was irrigated by 5 ml of distilled water and kept in a dark environment at a temperature of 20 °C for two days. Seedlings' growth is followed until they reach 4 days of germination and the number of seeds germinated is counted daily upon protrusion of the seed germ in each petri dish to calculate germination factors. The seedlings' germination continued for 14 days, six seedling samples were taken randomly from each dish to measure total shoot and root lengths. Lengths were measured with a Vernier caliper. The rice seedlings germination parameters as Germination potential, Germination rate, Germination index, and Vigor index are calculated as:
where, \(G_{n}\) = number of seedlings emerging on day ‘n’, and \(D_{n}\) = day ‘n’ after planting.
Proline Content Measurement
Proline content was determined according to the methodology of Bates et al. [27]. Fresh leaves (0.5 gm) were grinded to powder in a mortar with pestle, together with 10 ml of 3% (w/v) sulphosalicylic acid and the homogenate was filtered using Whatman No. 1 filter paper. Take 2.0 ml of the homogenate then add to it 2 ml of acid-ninhydrin and 2 ml glacial acetic acid and keep it in a boiling water bath for one hour then cool it in ice bath. Add 4 ml toluene to the homogenate and shake well for 30 s then let it settle down. Absorbance (Optical Density) at 520 nm was determined for toluene layer by carry series UV–Vis-NIR spectrometer then calculate the amount of proline present in the homogenate using proline standard curve.
Total Soluble Carbohydrates Measurement
Carbohydrates were determined according to the methodology of Dubios et al. [28]. Fresh leaves (1.0 gm) were grinded to powder in a mortar with pestle, together with 5 ml of 2.5 N hydrochloric acid and keeping it in a boiling water bath for three hours then cool it in room temperature and the homogenate filtered through filter paper. Take 0.1 ml of the homogenate then add to it 0.9 ml of distilled water. Add 1 ml of 5% phenol solution and 5 ml of concentrated sulfuric acid and keep at room temperature for 20 min. Absorbance (Optical Density) at 490 nm was determined by carry series UV–Vis-NIR spectrometer then calculate the amount of carbohydrates present in the homogenate using glucose standard curve.
Cellular Reactive Oxygen Species H2O2 Content Measurement
Cellular reactive oxygen species content was determined according to the methodology of Halliwell et al. 1987 [29]. Fresh leaves (1.0 gm) were grinded to powder in a mortar with pestle, together with 10 ml of 0.1% trichloroacetic acid and the homogenate filtered through filter paper in dark bottles. Take 0.5 ml of the homogenate then add to it 0.5 ml of 0.1 M phosphate buffer and 1 ml of 1 M potassium iodide in dark bottles and keep it in dark for 1 h. Absorbance (Optical Density) at 390 nm was determined by carry series UV–Vis-NIR spectrometer then calculate the amount of H2O2 present in the homogenate using H2O2 standard curve.
Lipid Peroxidation (MDA) Measurement
Lipid peroxidation level was estimated by malondiladehyde (MDA) that is a product of polyunsaturated fatty acid peroxide. The total MDA content was determined according to the methodology of Heath and Packer [30]. Fresh leaves (0.5 gm) were grinded to powder in a mortar with pestle, together with 10 ml of 0.1% trichloroacetic acid and the homogenate filtered through filter paper in dark bottles. Take 0.5 ml of the homogenate then add to it 1.5 ml of 0.5% thiobarbituric acid and keep it in a boiling water bath for fifteen minutes then cool it at room temperature. Absorbance (Optical Density) at 450, 532, 600 nm was determined by carry series UV–Vis-NIR spectrometer then calculate the amount of lipid peroxidation present in the homogenate.
Statistical Analysis
A fully randomized design was used to analyze experiential data with three replications for each plasma treatment time period (control: 0, 2, 5, 15, and 20 min). Measurements were expressed as mean value ± standard deviation (SD). Differences among mean values were analyzed by one-way analysis of variance (ANOVA), followed by Duncan’s multiple range tests (p < 0.05).
Results and Discussion
Discharge Characteristics
For direct DBD treatment of rice seeds with 4 mm air gap, Fig. 3a shows measured current and voltage characteristics (I-V). The discharge type is of filamentary mode characterized by several current peaks in the positive and negative half cycles corresponding to multiple micro-discharges.
The DBD power is calculated from the Lissajous figure in Fig. 3b as:
where Cd is the capacitance of dielectric, Cg is the capacitance of the gas, Vm is the maximum voltage attained after Qmax, V0 is the applied voltage, and f is the applied frequency [31].
From Fig. 3b for \(f=50 \mathrm{Hz},\) we can determine the values in Eq. 11 as:
By substitution in Eq. 11, we obtain the value of the DBD power to be 1.06 W for 4 mm air gap distance.
For indirect application with GAD-PAMi, Fig. 4 shows the current and voltage characteristics (I-V).
Power for GAD-PAMi can be calculated by the integration of instantaneous potential and current flow in a given time period as:
By using Eq. 12, we calculate the power P to be 4.23 W for 50 Hz discharge voltage. To compare the effects of the two devices described one should remember that the DBD acts on seeds placed in between the 4 mm air gap distance, while for the GAD-PAMi seeds are positioned 12 cm from the knife edges of the electrodes. For further investigations, we will assume that the application of direct and indirect plasma on rice seeds may be comparable considering that the effect of plasma generated at higher measured electrical power of GAD may be attenuated at larger application distance compared to the case of small application in the air gap distance in the DBD.
Effect of Plasma on Seed’s Temperature
The seed’s coat outside surface temperature was measured with an infrared sensor. A moderate increase in temperature occurred during DBD plasma treatment from a room temperature of 26.6 °C reaching a value around 30 °C, after 10 min of operation, as shown in Fig. 5. This is considered in an acceptable range not inducing seed’s morphological changes. For GAD-PAMi application, the temperature on seed’s coat is very close to room temperature due to water mist application.
Measurement of Water Mist Parameters During Plasma Applications with GAD-PAMi
After 20 min of operation of GAD-PAMi, we collect sufficient water mist by condensation on a suitable recipient to analyze water parameters. Changes occur in water parameters such as pH, temperature, conductivity, ORP, H2O2, nitrate and nitrite with plasma exposure as compared to distilled water parameters used to generate mist within the experiment. This is shown in Table 2.
With plasma exposure, pH value of water mist decreases largely exhibiting an acidity behavior. Conductivity and ORP of treated water increase moderately. The water temperature increases to 28.1 °C which is acceptable for the considered period. Nitrate increases largely after plasma exposure while nitrite increases moderately.
Optical Emission Spectroscopy (OES)
An emission spectrum of DBD in air over the range of 250 to 400 nm is shown in Fig. 6. Most of the distinct peaks obtained in the near-UV region corresponded to strong emissions from N2 species N2, N2 +, at 337.1, and 357.9 and OH excited species. An OH emission 309.8 nm is clearly observed. These results indicate that the DBD operated in the described conditions is a significant source of ROS/RNS.
Some O-containing and N-containing species are generated in the plasma discharge, as seen in the spectrum in Fig. 6. Those reactive species may be deposited on the seed’s coat and for the most energetic of them may have the possibility to penetrate to the seed bulk creating some changes in seed properties.
Contact Angle and Surface Energy of Seed’s Coating Surface
Contact angles and surface energy are calculated using Eqs. (1–5) for two liquids method. In Fig. 7 a and b we plot the values of contact angles on surface of rice seeds using two different liquids, distilled water, and glycerol as function of treatment time starting from untreated values for DBD and gliding arc operations respectively. The total surface free energy for direct and indirect operations are also plotted on Fig. 7a and b.
Contact angles of distilled water and glycerol drop on rice surface and total surface free energy of rice seeds at different treatment times a for DBD in air, b GAD-PAMi. Photos represent the water drop used for calculating contact angle (left photo for untreated and right one for 20 min treatment). For contact angle measurements with water and glycerol, one sample for each data point has been measured
The contact angles are found to decrease by 40.81 and 47.02% for distilled water, and by 25.84% and 31.7% by using glycerol after 20 min of DBD with air and GAD-PAMi, respectively. In comparison to the untreated seed value, total surface free energy is increased by 80.9 and 89.9% after 20 min of DBD with air and GAD-PAMi, respectively.
Effects of Plasma Treatment on Rice Seed Surface by SEM
The surface morphologies of rice seed coat for untreated, and DBD plasma treated samples were characterized by SEM, as shown in Fig. 8.
SEM images taken for plasma treated and non-treated seeds allowed assessing the seed surface structure modifications due to plasma exposure at different exposure time periods. The SEM images of untreated rice seeds show that the surface of rice seeds present aligned conical elements. Exposure of rice seeds to DBD plasma caused modifications of surface shape causing smoothing in the cone structure appearing in untreated seed surface. For larger exposure time periods some cracks appear. This can be due to the influence of reactive species formed in the plasma and reaching the seed coat causing plasma etching and surface functionalization [32].
FTIR Surface Analysis of Seed’s Surface
FTIR analysis was performed on seed coat after plasma treatment for increasing time periods for direct DBD application. FTIR measurements were made in an absorbance mode with a wavenumber range of 4000 to 800 cm−1 and a resolution of 4 cm−1.
Figure 9 shows the FTIR spectra of the seed coat. The range between 3600 and 2990 cm−1 were taken to refer to OH, the spectra between 3000 and 2600 cm−1 to refer to lipids, and the spectra between 1200 and 1000 cm−1 to refer to carbohydrates [33]. FTIR-ATR penetration depth (≈1.5 μm) much thinner than the thickness of the rice seed coat, one should assume that the possible changes observed by this technique are linked to the outer layers of the seed coat rather than the bulk of the seed. We can observe in Fig. 9 that OH, lipids, and carbohydrates increase with plasma treatment as compared to untreated samples. This increase is larger for indirect than for direct plasma exposure.
Measurements of Seeds Water Uptake
Figure 10a and b illustrate the seeds water uptake (imbibition) resulting after seeds exposure to DBD in air and GAD-PAMi as calculated by Eq. 6.
When compared with control seeds, the treated seeds have higher water uptake, which may be related to changes in seeds surface properties during plasma treatment by increasing hydrophilic wettability of the seed coat leading to accelerated water uptake. In Fig. 10, water uptake was observed to be higher during the first 2 h of imbibition compared to subsequent imbibition hours for which water uptakes are substantially lowered because seeds have reached water saturation. We can remark also from Figs. 10 a and b, that water uptake due to direct and indirect plasma are nearly comparable, except for the 20 min DBD direct exposure after 2 h where water content value reached its highest value. This can be explained by the existence of cracks on the seed coat as observed in SEM image of Fig. 8c. In indirect operation, due to activated mist, water is enabled to penetrate more easily across seed coat increasing water uptake, as seen in Fig. 10b.
Effects of Plasma Treatment of Rice Seeds on Germination Parameters and Seedling Growth
Germination potential was calculated on day 1 of germination using Eq. (7), germination rate and germination index were calculated at the end of the day 4 using Eqs. (8) and (9), and vigor index by Eq. (10). Results of rice seed germination exposed to plasma in direct and indirect operation using DBD in air and GAD-PAMi are presented in Tables 3 and 4 and plotted in Fig. 11 (from a to c) for time periods of 2, 5, 10, 15, and 20 min. Measurements used to calculate germination parameters were replicated three times and treated with the Statistical Package for the Social Sciences statistical analysis program in the form of mean value ± standard deviation.
Germination parameters a Germination potential, b Germination rate, and c Germination index for rice seeds treated with GAD-PAMi and DBD in air for different treatment time intervals. Each data point corresponds to an average value resulting from 3 samples. Each sample containing 52 seeds to be treated statistically
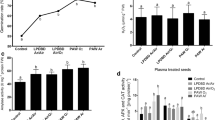
Exposure to direct DBD plasma and indirect GAD-PAMi had positive effects on the germination of rice seeds. We note that after 20 min plasma operation for direct and indirect exposures, values for germination potential increase compared to untreated case respectively by 36.73 and 50.4%, values of germination rate increase by 26.0 and 30.0%, and germination index increases by 25.92 and 36.53%. Results of germination parameters show superiority of gliding arc with mist treated seeds germination over control (untreated) seeds. After 14 days of seedlings' growth, the seedlings total shoot and root lengths of are measured. The results in Tables 5, 6 and Figs. 12(a to c) give the values for direct and indirect exposure for time periods of 2, 5, 10, 15, and 20 min as compared to untreated cases.
Seedlings' growth parameters a Total shoot length, b Total root length and c Vigor index for seedlings grown from seeds treated with GAD-PAMi and DBD in air for different treatment time intervals. Each data point corresponds to an average value resulting from 3 samples. Each sample containing 6 seeds to be treated statistically
After 20 min plasma operation for direct and indirect exposures for DBD in air and GAD-PAMi respectively, the total shoot length increases by 42.2 and 48.5%, the total root length increases by 15.93 and 22.42% and the vigor index increases by 44.46 and 55.86% in comparison with untreated samples. The characteristics of seedling growth have been improved as results of plasma treatment for both direct and indirect exposures as compared to untreated ones, this can be due to the insertions of oxygen and nitrogen hydrophilic functional groups that could have been formed on the seed’s surface by plasma. [34]. For indirect exposure through GAD-PAMi results indicate longer shoot and root lengths than direct exposure in the DBD. The vigor index of rice seeds is also increased with indirect plasma treatment. The enhancement in seed germination parameters can be related to hydrophilic behavior of seed’s coat and the increase in wettability caused by activated water mist which is enabled to penetrate more easily across seed coat increasing water uptake, as seen in Fig. 10b, which induce biochemical processes contributing to germination enhancement. Another important precursor for germination enhancement may be due to the increase in nitrate and nitrite values in activated mist as given in Table 2. This agrees with the finding of Sivachandiran and Khacef [35] that the higher germination rates obtained with plasma activated water (PAW) are due to increase in nitrate ion concentration. The effects of nitrate and nitrite on germination could be the response of antioxidant systems. Hendricks and Taylorson [36] suggested that its mechanism could be through a nitric oxide (NO) synthesis. NO breaks a seed dormancy through the interaction with the phytochrome signaling pathways, the ethylene biosynthesis, and interplays with ROS. Shen et al. [37] reported also that formation of important reactive species formed in PAW as ROS like atomic oxygen, singlet oxygen, superoxide, hydroxyl radicals and RNS like peroxynitrite, nitric oxide, nitrates, and nitrite ions play an important role in enhancing germination.
Evaluation of Some Physiological Parameters of Rice Seeds
In treated rice seeds, physiological parameters as free proline, total soluble carbohydrates, ROS, and MDA compared to control, are presented in Tables 7, and 8. As shown in Figs. 13a to d.
Plant physiological and biochemical results of rice seedlings from seeds treated with GAD-PAMi and DBD in air for different treatment time intervals. a Proline b Total Soluble Carbohydrates content c ROS content d MDA content. For each data point, we take 3 samples of 0.5 gm of green shoot per sample for proline and MDA, and 3 samples of 1.0 gm of green shoot per sample for carbohydrates and ROS, to be treated statistically
Plasma increased free proline, total soluble carbohydrate, and ROS while decreasing MDA in seedlings cultivated from treated rice seeds. In comparison with untreated samples, the free proline increased by 30.0 and 40.0% after 20 min of DBD with air and GAD-PAMi, respectively, shown in Fig. 13a and total soluble carbohydrates increased by 49.7 and 54.6% as shown in Fig. 13b. In comparison to the untreated samples, ROS increased by 52.0 and 60.0% after 20 min of DBD with air and GAD-PAMi, respectively, as in Fig. 13-c, and MDA decreased by 68.0 and 88.0% as shown in Fig. 13d. Proline and soluble sugar are considered as important substances contributing to osmotic protection of plants against abiotic conditions [38]. DBD direct treatment and GAP-PAMi as indirect treatment enhance the production of such quantities. The increase is more pronounced with activated mist exposure. MDA was generally measured as an indicator of membrane lipid peroxidation and membrane damage. MDA level was notably reduced after direct and indirect treatments. The reduction is more pronounced with larger exposure time [39].
The main benefit of cold plasma is its ability to produce reactive species in the form of ROS/RNS in the plasma treated media, either air or water vapor. During plasma direct or indirect seed exposures. ROS/RNS generated by plasma are deposited on seed coat and may migrate inside seed through outer surface cracks to probably initiate physical and chemical changes. This enables plasma, through the production of reactive species, to play an important role as signaling agents in plant cells by regulation of germination and plant physiology [40, 41]. The increased ROS/RNS produced by plasma in dry and germinating seeds induce internal seed cellular ROS followed by an increased activity of enzymes of antioxidant systems and compatible solutes, such as free proline and total soluble carbohydrates, which build up in leaves and roots of growing seedlings accompanied by a decreased levels of a lipid peroxidation marker malondialdehyde (MDA). Plasma seed treatment increases the tolerance of plants facing future exposure to biotic and abiotic stress agents, as excess of salt and soil dryness. So, plants will be prepared to respond to an increase in intracellular ROS levels due to its higher level of endogenous antioxidant defense activities produced by antioxidant enzymes initiated by seeds exposure to plasma either directly or indirectly.
Relation Between Proline and Cellular H2O2
From the results in Fig. 13, we can establish correlations between stress-induced proline accumulation and ROS in the form of cellular H2O2 production, as well as correlations between MDA and ROS. A positive correlation is found between free proline and ROS, and a negative correlation between MDA and ROS. This is shown in Figs. 14 a and b for direct plasma exposure and Fig. 15 a and b for indirect plasma exposure for different treatment time in the two cases.
Plants deal with abiotic stresses by production of ROS and Proline in plant cells. Proline acts as a nonenzymatic antioxidant in plant cells under abiotic stress and as a singlet oxygen quencher and scavenger of hydroxyl radicals. Proline may be important in preventing oxidative damage caused by ROS, it also stabilizes DNA, membranes and protein complexes, and provides a source of carbon and nitrogen for growth after stress relief [42]. The accumulated proline values, shown in Figs. 14a and 15a for direct and indirect plasma exposure, indicate a positive correlation with ROS, thus plants can adapt with abiotic stresses (caused by plasma in our case) because of the low content in MDA which exhibits negative correlation with ROS as shown in Figs. 14b and 15b for the two exposure schemes. One can observe that those effects are more pronounced for indirect GAD-PAMi than for direct DBD in air.
Summary and Conclusions
Exposures of rice seeds (Oryza sativa L.) for different time periods directly by DBD air plasma and indirectly by GAD-PAMi have enhanced rice seeds germination potential, germination rate, germination index, and seedlings growth of shoot and root lengths and vigor index. Many factors contribute to these seeds germination enhancement results such as the effect of plasma reactive species causing etching and inducing cracks on the seed coat. This leads to an improvement in seed water uptake and surface hydrophilicity by decreasing water contact angles and increasing seed surface free energy. In indirect method, increasing nitrate and nitrite of water mist by plasma may also help boosting germination parameters and seedlings growth of treated seeds. Plasma may also be involved in some changes inside the seed. Reactive species formed in the plasma may penetrate through the seed coat and migrate to seed bulk to initiate functional groups and cause physiological changes. We have observed in seedlings cultivated from plasma treated seeds an increase in proline cellular ROS and soluble carbohydrate, while a reduction of MDA (a lipid peroxidation product) accumulation. Plant deals with plasma as an abiotic stress, meanwhile plasma acts as elicitors for enhancement of germination and growth. It can be concluded that indirect exposure of rice seeds by plasma activated mist shows superior effects on germination parameters and seedling growth accompanied by some physiological changes of increasing nonenzymatic antioxidant substances, which in turn ameliorate the tolerance against abiotic stress. Plasma activated mist application in industrial scale could be more practical for large seed numbers treatment than direct plasma method.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Fróna D, Szenderák J, Harangi-Rákos M (2019) The challenge of feeding the world. Sustainability 11(20):5816
Raza A, Razzaq A, Mehmood SS, Zou X, Zhang X, Lv Y, Xu J (2019) Impact of climate change on crops adaptation and strategies to tackle its outcome: a review. Plants 8(2):34
Yadav S, Modi P, Dave A, Vijapura A, Patel D, Patel M (2020) Effect of abiotic stress on crops. IntechOpen. https://doi.org/10.5772/intechopen.88434
Hasanuzzaman M, Bhuyan MHMB, Zulfiqar F, Raza A, Mohsin SM, Mahmud JA, Fujita M, Fotopoulos V (2020) Reactive oxygen species and antioxidant defense in plants under abiotic stress: revisiting the crucial role of a universal defense regulator. Antioxidants 9(8):681
Huang H, Ullah F, Zhou DX, Yi M, Zhao Y (2019) Mechanisms of ROS regulation of plant development and stress responses. Front Plant Sci 10:800
Mittler R (2017) ROS are good. Trends Plant Sci 22(1):11–19
Dien DC, Mochizuki T, Yamakawa T (2019) Effect of various drought stresses and subsequent recovery on proline, total soluble sugar and starch metabolisms in Rice (Oryza sativa L.) varieties. Plant Prod Sci 22(4):530–545
Verbruggen N, Hermans C (2008) Proline accumulation in plants: a review. Amino Acids 35(4):753–759
Couée I, Sulmon C, Gouesbet G, El Amrani A (2006) Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J Exp Bot 57(3):449–459
Ayala A, Muñoz MF, Argüelles S (2014) Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-Hydroxy-2-nonenal. Oxid Med Cell Longev 2014:360438
Holubová Ľ, Kyzek S, Ďurovcová I, Fabová J, Horváthová E, Ševčovičová A, Gálová E (2020) Non-thermal plasma—A new green priming agent for plants. Int J Mol Sci 21(24):9466
Graves DB (2012) The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J Phys D: Appl Phys 45:263001
Seck PA, Diagne A, Mohanty S, Wopereis MCS (2012) Crops that feed the world 7: rice. Food Sec 4:7–24
Tan M, Xu J, Li F, Zhang C (2014) Physiological mechanisms of improving rice (Oryza sativa L.) seed vigor through arc-tooth-shaped corona discharge field treatment. AJCS 8(11):1495–1502
Teerakawanich N, Kasemsuwan V, Jitkajornwanich K, Kanokbannakorn W, Srisonphan S (2018) Microcorona discharge-mediated nonthermal atmospheric plasma for seed surface modification. Plasma Chem Plasma Process 38:817–830
Penado KNM, Mahinay CLS, Culaba IB (2017) Effect of atmospheric plasma treatment on seed germination of rice (Oryza sativa L.). Jpn J Appl Phys 57(1S):010AG8
Amnuaysin N, Korakotchakorn H, Chittapun S, Poolyarat N (2018) Seed germination and seedling growth of rice in response to atmospheric air dielectric-barrier discharge plasma. Songklanakarin J Sci Technol 40(4):819–823
Sheteiwy MS, An J, Yin M, Jia X, Guan Y, He F, Hu J (2019) Cold plasma treatment and exogenous salicylic acid priming enhances salinity tolerance of Oryza sativa seedlings. Protoplasma 256(1):79–99
Hashizume H, Kitano H, Mizuno H, Abe A, Yuasa G, Tohno S, Tanaka H, Ishikawa K, Matsumoto S, Sakakibara H, Nikawa S, Maeshima M, Mizuno M, Hori M (2021) Improvement of yield and grain quality by periodic cold plasma treatment with rice plants in a paddy field. Plasma Process Polym 18(1):2000181
Tanakaran Y, Matra K (2021) The influence of atmospheric non-thermal plasma on jasmine rice seed enhancements. J Plant Growth Regul 41:178–187
Rashid M, Rashid MM, Reza MA, Talukder MR (2021) Combined effects of air plasma seed treatment and foliar application of plasma activated water on enhanced paddy plant growth and yield. Plasma Chem Plasma Process 41:1081–1099
Rongsangchaicharean T, Srisonphan S, Onwimol D (2022) Responses of rice seed quality to large-scale atmospheric nonthermal plasmas. Plasma Chem Plasma Process 42:1127–1141
El Shaer M, Fayed H, Abd El-Hady HI, El Sebaei A, Mobasher M (2022) Impact of direct plasma jet and indirect plasma activated mist on surface properties of different material samples during bacterial inactivation. Plasma Med 12(3):23–40
Bracco G, Holst B (eds) (2013) Surface science techniques. Springer Science & Business Media, UK
Makkonen L (2016) Young’s equation revisited. J Phys Condens Matter 28(13):135001
Żenkiewicz M (2007) Methods for the calculation of surface free energy of solids. J Achiev Mater Manuf Eng 24(1):137–145
Bates LS, Waldren RA, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
DuBois M, Gilles KA, Hamilton JK, Rebers PT, Smith F (1956) Colorimetric method for determination of sugars and related substances. Anal Chem 28(3):350–356
Halliwell B, Gutteridge JM, Aruoma OI (1987) The deoxyribose method: a simple “test-tube” assay for determination of rate constants for reactions of hydroxyl radicals. Anal Biochem 165(1):215–219
Heath RL, Packer L (1968) Photoperoxidation in isolated chloroplasts: II. role of electron transfer. Arch Biochem Biophys 125(3):850–857
Wagner HE, Brandenburg R, Kozlov KV, Sonnenfeld A, Michel P, Behnke JF (2003) The barrier discharge: basic properties and applications to surface treatment. Vacuum 71(3):417–436
Waskow A, Howling A, Furno I (2021) Mechanisms of plasma-seed treatments as a potential seed processing technology. Front Phys 9:617345
Molina R, López-Santos C, Gómez-Ramírez A, Vílchez A, Espinós JP, González-Elipe AR (2018) Influence of irrigation conditions in the germination of plasma treated Nasturtium seeds. Sci Rep 8(1):16442
Gómez-Ramírez A, López-Santos C, Cantos M, García JL, Molina R, Cotrino J, Espinós JP, González-Elipe AR (2017) Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci Rep 7(1):5924
Sivachandiran L, Khacef A (2017) Enhanced seed germination and plant growth by atmospheric pressure cold air plasma: combined effect of seed and water treatment. RSC Adv 7(4):1822–1832
Hendricks SB, Taylorson RB (1974) Promotion of seed germination by nitrate, nitrite, hydroxylamine, and ammonium salts. Plant Physiol 54(3):304–309
Shen J, Tian Y, Li Y, Ma R, Zhang Q, Zhang J, Fang J (2016) Bactericidal effects against S. aureus and physicochemical properties of plasma activated water stored at different temperatures. Sci Rep 6(1):28505
Li Y, Wang T, Meng Y, Qu G, Sun Q, Liang D, Hu S (2017) Air atmospheric dielectric barrier discharge plasma induced germination and growth enhancement of wheat seed. Plasma Chem Plasma Process 37:1621–1634
Guo Q, Wang Y, Zhang H, Qu G, Wang T, Sun Q, Liang D (2017) Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci Rep 7(1):1–14
Smith JB, Adams I, Ji HF (2017) Biomolecule response to nonthermal plasma. Plasma Med 7(4):427–443
Mildaziene V, Ivankov A, Sera B, Baniulis D (2022) Biochemical and physiological plant processes affected by seed treatment with non-thermal plasma. Plants 11(7):856
Szabados L, Savouré A (2010) Proline: a multifunctional amino acid. Trends Plant Sci 15(2):89–97
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). No funding received.
Author information
Authors and Affiliations
Contributions
MES proposed the subject and wrote the manuscript. MA performed the germination and physiological measurements. HE contributed to experiment setup and electrical measurements. YH suggested the physiological measurements and explained the results. AA assisted data plotting. AZ performed electrical measurements. MM initiated research plan and suggested results discussions. All authors contributed to reviewing the original draft and approved the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Ethical Approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
El Shaer, M., Abdel-azim, M., El-welily, H. et al. Effects of DBD Direct Air Plasma and Gliding Arc Indirect Plasma Activated Mist on Germination, and Physiological Parameters of Rice Seed. Plasma Chem Plasma Process 43, 1169–1193 (2023). https://doi.org/10.1007/s11090-023-10350-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-023-10350-x