Abstract
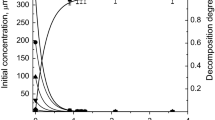
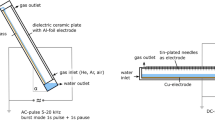
The results of studies of the decomposition of 2,4-dichlorophenol (2,4-DCP) in its aqueous solution under the action of atmospheric pressure DBD in an oxygen flow are presented. A new reactor design was used in which the discharge zone was filled with a sorbent (diatomite). It was found that the kinetics of decomposition obeys a first-order kinetic equation for the concentration of 2,4-DCP. The presence of an adsorbent significantly improves the parameters of the decomposition process. Decomposition rates, rate constants and energy efficiency are doubled. So, at a specific discharge power of 1.8 W/cm3 in the presence of a sorbent, the rate constant was ~1 s−1, and without it, ~0.5 s−1. The energy efficiency was 0.031 and 0.016 molecules per 100 eV, respectively. The parameters of the treated solution are improved in terms of its potential toxicity. The concentrations of the main decomposition products (aldehydes, carboxylic acids) in the presence of a sorbent are significantly less than without it. This is due to an increase in the rate of conversion of these products into carbon dioxide molecules. It was also shown that the decomposition of one 2,4-DCP molecule leads to the formation of two chloride ions in solution, and the ozone formed in the discharge does not significantly affect the destruction process.









Similar content being viewed by others
References
Hoseini SN, Pirzaman AK, Aroon MA, Pirbazari AE (2017) Photocatalytic degradation of 2,4-dichlorophenol by Co-doped TiO2 (Co/TiO2) nanoparticles and Co/TiO2 containing mixed matrix membranes. J Water Process Eng 17:124–134
Zhang C, Zhou M, Ren G, Yu X, Ma L, Yang J, Yu F (2015) Heterogeneous electro-Fenton using modified iron–carbon as catalyst for 2,4-dichlorophenol degradation: influence factors, mechanism and degradation pathway. Water Res 70:414–424
Jiang G, Lan M, Zhang Z, Lv X, Lou Z, Xu X, Dong F, Zhang S (2017) Identification of active hydrogen species on palladium nanoparticles for an enhanced electrocatalytic hydrodechlorination of 2,4-dichlorophenol in water. Environ Sci Technol 51(13):7599–7605
Simsek EB, Aytas B, Duranoglu D, Beker U, Trochimczuk AW (2016) A comparative study of 2-chlorophenol, 2,4-dichlorophenol, and 2,4,6-trichlorophenol adsorption onto polymeric, commercial, and carbonaceous adsorbents. Desalination Water Treat 57(21):9940–9956
Zhang H, Zhang Q, Miao C, Huang Q (2018) Degradation of 2,4-dichlorophenol in aqueous solution by dielectric barrier discharge: effects of plasma-working gases, degradation pathways and toxicity assessment. Chemosphere 204:351–358
Aziz KHH, Miessner H, Mueller S, Mahyar A, Kalass D, Moeller D, Khorshid I, Rashid MAM (2018) Comparative study on 2,4-dichlorophenoxyacetic acid and 2,4-dichlorophenol removal from aqueous solutions via ozonation, photocatalysis and non-thermal plasma using a planar falling film reactor. J Hazard Mater 343:107–115
Yang H, Caixia S, Tezuka M (2013) Plasma-induced decomposition of dichlorophenols and trichlorophenols in water by means of anodic contact glow discharge electrolysis. Plasma Chem Plasma Process 33(6):1043–1052
Gushchin AA, Grinevich VI, Kozlov AA, Kvitkova EY, Shutov DA, Rybkin VV (2017) Destruction of 2,4-dichlorophenol in an atmospheric pressure dielectric darrier discharge in oxygen. Plasma Chem Plasma Process 37(5):1331–1341
Bobkova ES, Rybkin VV (2015) Peculiarities of energy efficiency comparison of plasma chemical reactors for water purification from organic substances. Plasma Chem Plasma Process 35(1):133–142
Hao XL, Zhou MH, Zhang Y, Lei LC (2006) Enhanced degradation of organic pollutant 4-chlorophenol in water by non-thermal plasma process with TiO2. Plasma Chem Plasma Process 26(5):455–468
Qu GZ, Lu N, Li J, Wu Y, Li GF, Li D (2009) Simulataneous pentachlorophenol decomposition and granular activated carbon regeneration assisted by dielectric barrier discharge plasma. J Hazard Mater 172(1):472–478
Gushchin AA, Grinevich VI, Gusev GI, Kvitkova EY, Rybkin VV (2018) Removal of oil products from water using a combined process of sorption and plasma exposure to DBD. Plasma Chem Plasma Process 38(5):1021–1033
State Standard of RF 51209-98 (1998) Drinking water. Method for determining the content of organochlorine pesticides by gas-liquid chromatography, Ministry of Health of Russia (in Russian)
Simonov V, Nekhorosheva E, Zavorovskaya N (1988) Analysis of the air during the processing of polymeric materials. Khimiya, Moscow
Parkinson WH, Yoshino K, Freeman DE (1988) Absolute absorption cross section measurements of ozone and the temperature dependence at four reference wavelengths leading to renormalization of the cross section between 240 and 350 nm. Smithsonian Institution Astrophysical Observatory, Cambridge, p 02138
Bobkova ES, Khodor YaV, Kornilova ON, Rybkin VV (2014) Chemical composition of plasma of dielectric barrier discharge at atmospheric pressure with a liquid electrode. High Temp 52(4):511–517
Gushchin AA, Grinevich VI, Shulyk VYa, Kvitkova EYu, Rybkin VV (2018) Destruction kinetics of 2,4-dichlorophenol aqueous solutions in an atmospheric pressure dielectric barrier discharge in oxygen. Plasma Chem Plasma Process 38(1):123–134
Kovačević VV, Dojčinović DP, Jović M, Roglić GM, Obradović BM, Kuraica MM (2017) Measurement of reactive species generated by dielectric barrier discharge in direct contact with water in different atmospheres. J Phys D Appl Phys 50(15):155205
Kogelschatz U (2003) Dielectric-barrier discharges: their history, discharge physics, and industrial applications. Plasma Chem Plasma Process 23(1):1–46
Acknowledgements
This study was carried out in the frame of State Assignment of the Ministry of Education and Science of the RF, No. FZZW-2020-0009 and No. FZZW-2020-0010 and it was supported by the RFBR Grant, Project No. 18-08-01239 A.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gushchin, A.A., Gusev, G.I., Grinevich, V.I. et al. Destruction of 2,4-Dichlorophenol in Water Solution Using a Combined Process of Sorption and Plasma Exposure to DBD. Plasma Chem Plasma Process 41, 421–431 (2021). https://doi.org/10.1007/s11090-020-10132-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10132-9