Abstract
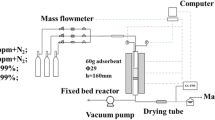
In this paper, a nanosecond pulsed discharge was employed to modify activated carbon (AC) adsorbents for the purpose of detecting gas-phase polycyclic aromatic hydrocarbons (PAHs). The raw and modified AC were characterized by helium ion microscopy, N2 adsorption/desorption, and X-ray photoelectron spectroscopy. The treatment time, gas composition, and pulse peak voltage of discharge were optimized to improve the adsorption capacity of AC. And the adsorption kinetics of AC was investigated. It is found that the pore structure of AC is changed and the oxygen-containing groups on AC surface are increased after plasma treatment. As a result, the roles of physisorption and chemisorption are promoted, and the adsorption rate of naphthalene is improved by about 10%. More importantly, modified AC adsorbing PAHs obeys Pseudo-first-order model and Langmuir isotherm model, and the corresponding adsorption coefficients are fitted, which contributes to detecting PAHs more accurately. After modified AC enriching, the gas-phase PAHs can be detected with the limit of detections in the range of 20–120 μg/m3.








Similar content being viewed by others
References
Kim K, Jahan SA, Kabir E, Brown RJC (2013) A review of airborne polycyclic aromatic hydrocarbons (PAHs) and their human health effects. Environ Int 60:71–80
Lamichhane S, Bal Krishna KC, Sarukkalige R (2016) Polycyclic aromatic hydrocarbons (PAHs) removal by sorption: a review. Chemosphere 148:336–353
Weschler CJ, Nazaroff WW (2008) Semivolatile organic compounds in indoor environments. Atmos Environ 42:9018–9040
Wu ZL, Zhu ZB, Hao XD, Zhou WL, Han JY, Tang XJ, Yao SL, Zhang XM (2018) Enhanced oxidation of naphthalene using plasma activation of TiO2/diatomite catalyst. J Hazard Mater 347:48–57
Huo SH, An HY, Yu J, Mao XF, Zhang Z, Bai L, Huang YF, Zhou PX (2017) Pyrolytic in situ magnetization of metal-organic framework MIL-100 for magnetic solid-phase extraction. J Chromatogr A 1517:18–25
Pandey SK, Kim KH, Brown RJC (2011) A review of techniques for the determination of polycyclic aromatic hydrocarbons in air. TrAC Trends Anal Chem 30:1716–1739
Poster DL, Schantz MM, Sander LC, Wise SA (2006) Analysis of polycyclic aromatic hydrocarbons (PAHs) in environmental samples: a critical review of gas chromatographic (GC) methods. Anal Bioanal Chem 386:859–881
Fresco-Cala B, Cárdenas S, Valcárcel M (2016) Preparation and evaluation of micro and meso porous silica monoliths with embedded carbon nanoparticles for the extraction of non-polar compounds from waters. J Chromatogr A 1468:55–63
Nika CE, Yiantzi E, Psillakis E (2016) Plastic pellets sorptive extraction: low-cost, rapid and efficient extraction of polycyclic aromatic hydrocarbons from environmental waters. Anal Chim Acta 922:30–36
Haruna K, Saleh TA, Hossain MK, Al-Saadi AA (2016) Hydroxylamine reduced silver colloid for naphthalene and phenanthrene detection using surface-enhanced Raman spectroscopy. Chem Eng J 304:141–148
Tommasini M, Lucotti A, Alfè M, Ciajolo A, Zerbi G (2016) Fingerprints of polycyclic aromatic hydrocarbons (PAHs) in infrared absorption spectroscopy. Spectrochim Acta Part A Mol Biomol Spectrosc 152:134–148
Luna FMT, Pontes Filho AA, Trindade ED, Cavalcante CL (2016) Rapid assessment of total and polycyclic aromatic contents in heavy oils. Environ Monit Assess 188:215
Polycyclic Aromatic Hydrocarbons (PAHs) Fact Sheet (2008) USEPPA, Washington DC https://www.epa.gov/north-birmingham-project/polycyclic-aromatic-hydrocarbons-pahs-fact-sheet
WHO (2003) Polynuclear aromatic hydrocarbons in drinking water. WHO, Geneva
Helaleh MIH, Al-Omair A, Nisar A, Gevao B (2005) Validation of various extraction techniques for the quantitative analysis of polycyclic aromatic hydrocarbons in sewage sludges using gas chromatography-ion trap mass spectrometry. J Chromatogr A 1083:153–160
Cheng XK, Kan AT, Tomson MB (2004) Naphthalene adsorption and desorption from aqueous C 60 fullerene. J Chem Eng Data 49:675–683
Zhang J, Duang Y, Zhou Q, Zhu C, She M, Ding WK (2016) Adsorptive removal of gas-phase mercury by oxygen non-thermal plasma modified activated carbon. Chem Eng J 294:281–289
Li ZY, Liu YS, Yang X, Xing Y, Wang ZY, Yang Q, Yang RT (2015) Desorption kinetics of naphthalene and acenaphthene over two activated carbons via thermogravimetric analysis. Energy Fuel 29:5303–5310
Wu L, Wan WJ, Shang ZS, Gao XY, Kobayashi N, Luo GQ, Li ZY (2018) Surface modification of phosphoric acid activated carbon by using non-thermal plasma for enhancement of Cu(II) adsorption from aqueous solutions. Sep Purif Technol 197:156–169
Wang J, Chen ZM, Chen BL (2014) Adsorption of polycyclic aromatic hydrocarbons by graphene and graphene oxide nanosheets. Environ Sci Technol 48:4817–4825
Bhatnagar A, Hogland W, Marques M, Sillanpää M (2013) An overview of the modification methods of activated carbon for its water treatment applications. Chem Eng J 219:499–511
Shaarani FW, Hameed BH (2011) Ammonia-modified activated carbon for the adsorption of 2,4-dichlorophenol. Chem Eng J 169:180–185
Tchomgui-Kamga E, Alonzo V, Nanseu-Njiki CP, Audebrand N, Ngameni E, Darchen A (2010) Preparation and characterization of charcoals that contain dispersed aluminum oxide as adsorbents for removal of fluoride from drinking water. Carbon 48:333–343
Zhang W, Liu HY, Xia QB, Li Z (2012) Enhancement of dibenzothiophene adsorption on activated carbons by surface modification using low temperature oxygen plasma. Chem Eng J 209:597–600
Yuan H, Wang WC, Yang DZ, Zhou XF, Zhao ZL, Zhang L, Wang S, Feng J (2018) Hydrophilicity modification of aramid fiber using a linear shape plasma excited by nanosecond pulse. Surf Coat Technol 344:614–620
Yuan H, Wang WC, Yang DZ, Zhao ZL, Zhang L, Wang S (2017) Atmospheric air dielectric barrier discharge excited by nanosecond pulse and AC used for improving the hydrophilicity of aramid fibers. Plasma Sci Technol 19:125401
Zhang L, Yang DZ, Wang WC, Wang S, Yuan H, Zhao ZL, Sang CF, Jia L (2016) Needle-array to plate DBD plasma using sine AC and nanosecond pulse excitations for purpose of improving indoor air quality. Sci Rep 6:25242
Kuroki T, Oishi T, Yamamoto T, Okubo M (2013) Bromomethane decomposition using a pulsed dielectric barrier discharge. IEEE Trans Ind Appl 49:293–297
Duan SX, Xu XT, Liu X, Wang YN, Hayat T, Alsaedi A, Meng YD, Li JX (2018) Highly enhanced adsorption performance of U(VI) by non-thermal plasma modified magnetic Fe3O4 nanoparticles. J Colloid Interface Sci 513:92–103
Kossyi IA, Kostinsky AY, Matveyev AA, Silakov VP (1992) Kinetic scheme of the non-equilibrium discharge in nitrogen-oxygen mixtures. Plasma Sources Sci Technol 1:207–220
Sun J, Liu X, Zhang FS, Zhou J, Wu J, Alsaedi A, Hayat T, Li JX (2019) Insight into the mechanism of adsorption of phenol and resorcinol on activated carbons with different oxidation degrees. Colloid Surfaces A 563:22–30
Pan J, Li L (2015) Particle densities of the pulsed dielectric barrier discharges in nitrogen at atmospheric pressure. J Phys D Appl Phys 48:055204
Auta M, Hameed BH (2011) Optimized waste tea activated carbon for adsorption of methylene blue and acid blue 29 dyes using response surface methodology. Chem Eng J 175:233–243
Azizian S (2004) Kinetic models of sorption: a theoretical analysis. J Colloid Interface Sci 276:47–52
Acknowledgements
This work is supported by National Key R&D Project (2016YFC0207200), and the National Natural Science Foundations of China (Grant Nos. 51677019, 51977023), Science and Technology Development Fund of Xinjiang Production and Construction (2019BC009), Fundamental Research Funds for the Central Universities (DUT18LK42), and Fund of Science and Technology on High Power Microwave Laboratory (JCKYS2018212036).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, H., Yang, D., Jia, Z. et al. Activated Carbon Modified by Nanosecond Pulsed Discharge for Polycyclic Aromatic Hydrocarbons Detection. Plasma Chem Plasma Process 40, 1539–1553 (2020). https://doi.org/10.1007/s11090-020-10114-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-020-10114-x