Abstract
The reduction of high concentrations of NOX and SO2 has been studied for the simulation of NOX and SO2 removal from off-gases generated by diesel engines in which heavy fuel oil is usually used. The combination of electron beam (EB) and a wet scrubber method proved to be very promising for this purpose. In this work, we investigated NOX reduction under EB with the application of NaClO, NaClO2, or NaClO3 as an oxidant in two different scrubber solutions, phosphate buffer (Na2HPO4–KH2PO4)–simulated sea water solution and NaOH–simulated sea water solution. The concentration of buffer in the scrubber solution was 5 mM. The concentration of NaOH in the scrubber solution was 7 mM. Compared with the removal performance of NO for the three different oxidants, the application of NaClO2 gave the best removal efficiency. For the inlet concentration of NO gas set at 1000 ppm and the EB dose set at 10.9 kGy, the application of NaClO2 reaches a maximum of 95.03% NO removal efficiency, while the NaClO oxidant reached a maximum of 49.84% removal efficiency and the NaClO3 oxidant reached a maximum of 54.25% removal efficiency. The results show that the chosen series of oxidants have the potential to be applied in the removal of NOX and SO2 from the off-gases of a diesel engine. For example, they could be used on cargo ships, which generate large amounts of NOX and SO2 from the combustion of heavy oil which contains sulphur and nitrogen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acid rain, global warming, ozone depletion, and smog are preeminent environmental problems facing the world today. Nitrogen oxides (NOX) and sulphur dioxide (SO2), produced primarily by coal-burning power plants and automobile exhaust, are major contributors to acid rain [1]. Carbon dioxide, which is released when fossil fuels like coal, oil, and natural gas are burned, is the biggest contributor to the accumulating greenhouse effect. Volatile organic compounds (VOCs) have high vapor pressure at room temperature due to their low boiling point. Some VOCs are dangerous to human health or cause harm to the environment and they are present in fumes given off by gasoline [2]. As the main threat to the ozone layer, VOCs produce the chemicals that could lead to global warming as well.
For the removal of these mentioned toxic gases such as NOX, SO2, and VOCs, although well-understood conventional technologies do exist for the treatment of these toxic gases, they have their own limitations in the aspect of cost, energy requirements, and by-product disposal. Non-thermal plasma techniques such as electron beam (EB) irradiation technology offer the advantages of energy efficiency and the capability for the simultaneous removal of coexisting pollutants [3]. The high energy electrons from the electron beam cause ionization and dissociation of air pollutants; the primary species (including excited species, radicals etc.) and secondary electrons are generated. These primary species and thermalized secondary electrons react with the toxic components to decompose them into less harmful products.
For the removal of SO2 only, the wet scrubber method is very popular due to the good solubility of SO2. Due to the insolubility of NO, whose concentration is over 90% of NOX, the conventional wet scrubber disposal method for off-gas such as SO2 could not be applied directly for the removal of NOX. Electron beam flue gas treatment (EBFGT) has been successfully applied for the removal of SO2 and NOX from flue gas emitted from an industrial electrical power station in Pomorzany, Poland [4]. In this process, ammonia was added into flue gas to removes H2SO4 and HNO3 (oxidation products of SO2 and NOx under EB irradiation) by heterogeneous reactions. A mixture of ammonia sulphate and ammonia nitrate was formed and removed from flue gas using electrostatic precipitator (ESP).
Under an EB, NO is first oxidized into NO2 which has a good solubility and can be easily removed from gas stream using wet-scrubber methods [5]. Therefore, the combination of EB and wet scrubber methods has been tested for the removal of coexisting high concentrations of NOX and SO2 from flue gases. Our preliminary results show that, compared with the case of the single application of EB, NOX removal efficiency increased when the hybrid system (EB combined with wet-scrubber) was used and where simulated sea water was used as wet scrubber solution. In this work we studied SO2 and NO removal using EB and a one stage wet scrubber system. Different scrubber solutions, such as phosphate buffer (Na2HPO4–KH2PO4)–simulated sea water solution and NaOH–simulated sea water solution, with addition of different oxidants such as NaClO, NaClO2, or NaClO3 were tested.
Experimental Method
Chemicals
The following chemical reagents were used to make scrubber solutions: NaCl (solid, sodium chloride, Purity of NaCl is ≥ 99.8%, Ca content is about 0.008% (wt/wt), CHEMPUR, Poland), NaClO (liquid, sodium hypochlorite solution (6–14% active chlorine), density 1.22–1.25 g/cm3), NaClO2 (solid, sodium chlorite, ACS reagent), NaClO3 (solid, sodium chlorate, ACS reagent), and NaOH (solid, sodium hydroxide, ACS reagent) purchased from Sigma Aldrich, USA; Na2HPO4 (anhydrous solid disodium hydrogen phosphate, ACS reagent) and KH2PO4 (solid, potassium dihydrogenophosphate, ACS reagent), purchased from VWR CHEMICALS, USA.
Irradiation
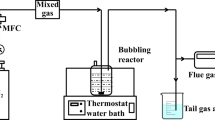
All experiments were carried out at the installation of the Institute of Nuclear Chemistry and Technology in Warsaw. The set-up of the installation was the same as described by Licki et al. [6].
The series of experiments was carried out using the accelerator ILU-6M (2 MeV, maximum beam power up to 20 kW). Cellulose triacetate (CTA) dosimeter was applied to measure the applied dose and 10.9 kGy dose was applied in the experiments.
Preparation of Flue Gases and Wet Scrubber Solutions
The simulated sea water (3.5% w/w NaCl solution) was prepared by dissolving a certain amount of sodium chloride in distilled water. 0.37 mL of the original NaClO stock solution as oxidant was added to the total 1.2 L aqueous scrubber solution. Similarly, in order to prepare NaClO2–sea water wet scrubber solution, 0.6 g NaClO2 was added to the total 1.2 L aqueous solution. For the preparation of NaClO3–sea water wet scrubber solution, 0.7 g NaClO3 powder was added to the total 1.2 L aqueous solution. The prepared 1.2 L solution was kept in the two scrubbers (Scrubber I and Scrubber II) connected in series; each scrubber contained 600 mL of scrubber solution. Two kinds of the scrubber solution were used, one was simulated sea water–phosphate buffer (Na2HPO4 and KH2PO4) and the other was simulated sea water–NaOH.
The simulated flue gases were generated by burning Polish light fuel oils from an oil burner. NOX concentration in the combustion flue gas was lower than the concentrations studied in the experiment. Additional NO from NO gas cylinders was added into the flue gas and the desired concentration of NO was regulated by a gas flow meter.
The small amount of flue gas (less than 200 mL/h) after irradiation was passed through the two scrubbers. The rest of the flue gas was discharged into the atmosphere through a stack after the retention chamber.
Each condition of the experiment was repeated at least twice, average value was reported.
Analysis Methods
The concentrations of SO2 and NOX before and after the process were analysed by a LANCOM series II Portable Emissions Analyzer (LAND combustion company, UK). The analysis of the scrubber solutions before and after process of NOX and SO2 removal was done by Ion Chromatography (DIONEX 2000 i/SP, USA). The removal efficiency of the flue gas (R%) is denoted by the following equation, where C1 denotes the initial concentration of a certain compound of flue gas and C2 denotes the concentration of a certain compound of flue gas after the clean-up procedure.
Results and Discussion
EB Combined Wet Scrubber System: Sea Water–Phosphate Buffer with Different Oxidant Additions
NO reduction was first investigated in the EB combined with the wet scrubber system with NaClO–sea water–buffer as the wet scrubber solution. The flue gas was first irradiated with EB. This was followed by the wet scrubber process. Figure 1 shows the whole process during EB treatment (first 13 min) and EB with the wet scrubber (17–25 min). Sea water–NaClO–buffer (Na2HPO4 and KH2PO4) was applied as the wet scrubber solution. It shows that NOX removal efficiency was about 15.0% under sole EB irradiation. When the wet scrubber was applied just after EB irradiation, NOX removal efficiency increased from 15.0 to 43.68%. The pH of the scrubber solution was reduced from 6.290 to 5.932 (Scrubber I) and 6.028 (Scrubber II) after the treatment. With the same dose of EB at 10.9 kGy, the introduction of combined EB and wet scrubber technology increased the removal efficiency of SO2 from 32.69 to 99.72%, while the single application of EB only gave 32.69% removal efficiency. In this process, the initial concentration of SO2 was 722 ppm.
NO reduction was further investigated in the EB with the wet scrubber system with addition of the oxidant NaClO2. Figure 2 shows the whole process during EB treatment (first 11 min) and EB with the wet scrubber (12–33 min). Sea water–NaClO2–buffer (Na2HPO4 and KH2PO4) was applied as the wet scrubber solution. The inlet concentration of NO was 1046 ppm. It is seen that NOX removal efficiency was about 19.21% under sole EB irradiation. When the wet scrubber was applied just after EB irradiation, NOX removal efficiency increased from 19.21 to 95.03%. The pH of the scrubber solution was reduced from 6.264 to 6.176 (Scrubber I) and 6.073 (Scrubber II) after the treatment. With the same dose of EB at 10.9 kGy, the introduction of combined EB and wet scrubber technology increased the removal efficiency of SO2 from 28.55 to 98.93%, while the single application of EB only gave 28.55% removal efficiency. In this process, the initial concentration of SO2 was 746 ppm.
NO reduction was investigated in the EB with the wet scrubber system with NaClO3 addition. Figure 3 shows the whole process during EB treatment (first 13 min) and EB with the wet scrubber (15–25 min). Sea water–NaClO3–buffer (Na2HPO4 and KH2PO4) was applied as the wet scrubber solution. Inlet concentration of NO was 1151 ppm. It is seen that NOX removal efficiency was about 15.33% under sole EB irradiation. When the wet scrubber was applied just after EB irradiation, NOX removal efficiency increased from 15.33 to 54.25%. The pH of the scrubber solution was reduced from 6.141 to 5.979 (Scrubber I) and 6.073 (Scrubber II) after the treatment. With the same dose of EB at 10.9 kGy, the introduction of combined EB and wet scrubber technology increased the removal efficiency of SO2 from 28.59 to 99.72%, while the single application of EB only gave 28.59% removal efficiency. In this process, the initial concentration of SO2 was 745 ppm.
EB Combined Wet Scrubber System: Sea Water–NaOH with Different Oxidant Additions
The EB/absorption system for NOX removal was further studied. NaOH replaced the phosphate buffer in the wet scrubber solution; a 10.9 kGy dose was applied and the NaOH concentration was about 7 mM. The initial concentration of NOX was about 1214 ppm. The results are presented in Fig. 4 when NaClO–sea water–NaOH was used as wet scrubber solution. It is seen that NOX removal efficiency was about 15.0% under sole EB irradiation (5–15 min). When the wet scrubber was applied just after EB irradiation, NOX removal efficiency increased from 15.0 to 46.62% (22–32 min). The pH of the scrubber solution was reduced from 11.487 to 6.214 (Scrubber I) and 6.556 (Scrubber II). After the treatment, the post process solution might be directly discharged. With the same dose of EB at 10.9 kGy, the introduction of combined EB and wet scrubber technology increased the removal efficiency of SO2 from 27.84 to 99.48%, while the single application of EB only gave 27.84% removal efficiency. In this process, the initial concentration of SO2 was 776 ppm.
The EB/absorption system for NOX removal was further studied with the addition of the oxidant NaClO2 in the wet scrubber solution. NaOH replaced the buffer in the wet scrubber system; 10.9 kGy dose was applied and NaOH concentration was about 7 mM. The initial concentration of NOX was about 1091 ppm. The results are presented in Fig. 5. It is seen that NOX removal efficiency was about 20.35% under sole EB irradiation (1–16 min). When the wet scrubber was applied just after EB irradiation, NOX removal efficiency increased from 20.35 to 92.67% (17–39 min). The pH of the scrubber solution was reduced from 11.039 to 6.128 (Scrubber I) and 6.573 (Scrubber II) after the treatment. With the same dose of EB at 10.9 kGy, the introduction of combined EB and wet scrubber technology increased the removal efficiency of SO2 from 27.31 to 98.23%, while the single application of EB only gave 27.31% removal efficiency. In this process, the initial concentration of SO2 was 736 ppm.
The hybrid plasma and chemical system for the removal of NOX and SO2 was applied when the NaClO3–NaOH–sea water wet scrubber was selected for the decontamination process; a 10.9 kGy dose was applied and NaOH concentration was about 7 mM. The initial concentration of NOX was about 1054 ppm. The results are presented in Fig. 6. It is seen that NOX removal efficiency was about 20.21% under sole EB irradiation (1–15 min). When the wet scrubber was applied just after EB irradiation, NOX removal efficiency increased from 20.21 to 47.15% (16–30 min). The pH of the scrubber solution was reduced from 10.984 to 6.097 (Scrubber I) and 6.451 (Scrubber II) after the treatment. With the same dose of EB at 10.9 kGy, the introduction of combined EB and wet scrubber technology increased the removal efficiency of SO2 from 30.05 to 99.87%, while the single application of EB only gave 30.05% removal efficiency. In this process, the initial concentration of SO2 was 742 ppm.
Comparison of Removal Effects Between Different Wet Scrubber Systems
A comparison of the removal performance in the EB–wet scrubber system with the application of three different types of oxidants such as NaClO, NaClO2, and NaClO3 for the removal of NOX and SO2 from simulated flue gases is shown in the Fig. 7 (for sea water–buffer solution) and in Fig. 8 (for sea water–NaOH solution), respectively. In the sea water–buffer (Na2HPO4–KH2PO4) solution, the removal efficiency of SO2 reached 99.72%, 98.93% and 99.72%, the removal efficiency of NOX reached 43.69%, 95.03%, and 54.25% at the dose of 10.9 kGy when NaClO, NaClO2 and NaClO3 were added into the sea water–phosphate buffer scrubber solutions, respectively; In the sea water–NaOH aqueous solution, the removal efficiency of SO2 reached 99.48%, 98.23% and 99.78%, the removal efficiency of NOX reached 49.84%, 92.67%, and 47.15% at the dose of 10.9 kGy when NaClO, NaClO2, and NaClO3 were added into sea water–NaOH scrubber solution, respectively. NaClO2 exhibited the highest performance for NOX removal in both cases. However SO2 removal efficiency was very high in all of these conditions (Table 1).
According to the two-film theory of gas–liquid reaction, a higher concentration of oxidant would enhance the mass transfer and promote the reaction in the liquid phase, resulting in an increase in NO absorption efficiency [7]. In this work, the same concentration of oxidants, such as NaClO, NaClO2 and NaClO3, was used to avoid the potential concentration effects on the NO absorption efficiency. Due to this point, the other aspects of the reaction kinetics are key for the removal efficiency of NOX. According to basic principles of physical chemistry, the chemical removal/absorption kinetic mechanism of the oxidant NaClO2 for NOX removal is a fast parallel reaction [7] in which several ongoing chain reactions occur at almost the same time.
Therefore, it is convenient and easier for the NaClO2 assisted removal/absorption process to reach high efficiency. The reactions for the application of NaClO could be denoted by the following chemical reactions [8].
At the same time, the reactions for the application of NaClO3 could be denoted by the following chemical reactions [9].
The chlorate ion (ClO3−) from chloric acid (HClO3) has unique oxidization chemical properties, especially in its acid form (HClO3) [8]. HClO3 has a higher oxidation potential than chlorine under most conditions due to one of its main reaction chemical intermediates-chlorine dioxide (ClO2). For the application of air pollution control technology, such as combined NOX and SOX removal from waste off-gases generated by combustion and chemical process, the application of chlorate ion (ClO3−) is usually preferred in its acid form (HClO3) rather than sodium chlorate (NaClO3) in which the addition of extra acid HClO3 would improve the removal efficiency [9]. Therefore, the application of NaClO3 in this study does not obtain the same removal efficiency of NO that the NaClO2 oxidant does. Comparing the oxidation ability of the chosen three ions, due to the weaker oxidation ability of hypochlorite ion (ClO−) than the other two, the application of the NaClO oxidant system did not give the same removal performance effect as the most promising NaClO2 oxidant. However, a higher concentration of the reaction reagent, for example oxidants which are applied, would enhance mass transfer and promote the reaction in the liquid phase, thus resulting in an increase in NO absorption efficiency. It is expected that the increasing concentration of the applied NaClO and NaClO3 would also have the potential of bringing about better removal efficiency of NO. Therefore, NaClO, NaClO2, and NaClO3 oxidants have the potential to find their own application for the removal of NOX and SO2 from diesel engine flue gases (e.g., on a cargo ship).
Influence of Inlet Concentration of NO on the NOX Removal Performance
The influence of different inlet concentrations of NO on NOX removal efficiency was studied with the addition of these two oxidants, NaClO2 or NaClO3, into the scrubber solutions. Two different wet scrubber solutions were used; one was sea water–phosphate buffer and the other was sea water–NaOH. Figure 9 shows NO removal efficiency with different inlet concentrations of NO when sea water–buffer–NaClO2 wet scrubber solution was applied for the purpose of the clean-up process. When the inlet concentration of NO increased from 568 to 1046 ppm, the total NOX removal efficiency increased from 93.49 to 95.03%. This increasing trend was also seen when sea water–NaOH–NaClO2 was applied as the wet scrubber aqueous solution (Fig. 10). With increasing the NO concentration from 282, 598, and 1091 to 1515 ppm, the NOX removal efficiency increased from 80.14, 92.14, and 92.67 to 93.17%. With the application of NaClO2 in the wet scrubber solution, the increase of inlet NO concentration improves the removal performance of NOX. The inlet concentration of NO for the chosen amount of NaClO2 oxidant has not yet reached the oxidation limitation for the amounts of NaClO2 used.
When the oxidant NaClO3 was applied in the wet scrubber system, a different phenomenon of NOX removal efficiency versus inlet concentration of NO was observed for NaClO3 and NaClO2. In the sea water–buffer–NaClO3 aqueous solution, when the inlet concentration of NO increased from 1151 to 1510 ppm, the total removal efficiency of NOX decreased from 54.25 to 42.47% (Fig. 11). The similar trend was observed when the sea water–NaOH–NaClO3 wet scrubber aqueous solution was applied (Fig. 12). With increasing the NO inlet concentration from 228, 631, and 1054 to 1583 ppm, the removal efficiency of NOX decreased from 88.16 to 60.54, 47.15, and 41.09%, respectively. The decreasing removal performance of NOX with increasing the concentration of NO is plausible due to the limited oxidation ability of NaClO3.
Product Analysis
Ion chromatography was used to analyse the post process solution using the EB–wet scrubber system. The experimental conditions of the inlet concentration of NO was 1515 ppm, the irradiation dose was 10.9 kGy, and sea water–NaOH–NaClO2 was used as the wet scrubber solution. For comparison, pure water was used as the wet scrubber solution to treat NO flue gas at the same dose of 10.9 kGy and with the inlet concentration of NO at 1392 ppm, which was close to 1515 ppm when the sea water–NaOH–NaClO2 wet scrubber was applied. Two cations including (Na+ and Ca2+) and three anions (NO3−, NO2−, and Cl−) were measured. The analysis results are shown in Table 2. It shows that the concentrations of NO2− and NO3− were increased in the sea water–NaOH–NaClO2 wet scrubber system compared with pure water as the scrubber solution. During the EB irradiation process, the application of the EB results in the oxidation of NO to NO2, which then transforms into HNO2 and HNO3 in aqueous solution. Due to this point, large amounts of the NO3− anion exist in the aqueous solution. From Table 2, it is also seen that the concentrations of NO2− and NO3− increased markedly in the sea water–NaOH–NaClO2 wet scrubber system compared with pure water as the scrubber solution. This indicates that NaClO2 has also participated in the process of oxidizing NO into NO2 and NO2 into NO3−. Comparing the two series of ion concentration analysis results, the increase in the concentration of Na+ and Cl− in aqueous solution is due to the application of a large amount of reagents containing these two ions. Due to impurity of sodium chloride, where 0.008% Ca is presence, it causes the increase in the concentration of Ca2+ from 2 (mg/L) to 11.7 (mg/L) when NaCl was added into scrubber solution.
Conclusions
The removal of high concentrations of NOX from flue gas was studied under EB and EB–hybrid systems with NaClO, NaClO2, and NaClO3 addition. It was seen that NOX removal efficiency apparently increased when an EB–hybrid system was applied. For example, the efficiency increased from 15.0 to 43.68% with NaClO–phosphate buffer–sea water or to 46.62% with NaClO–NaOH–sea water, if the oxidant NaClO was applied. For the case of applying the oxidants NaClO2 or NaClO3, the trends were similar. That is, better performance for the removal of NOX was observed with the combined application of EB with wet scrubber technology. The application of NaClO2 reached the highest removal record for NOX in this study. For the application of NaClO2–phosphate buffer–sea water system, the total removal efficiency for NOX reached 95.03%. For the application of the NaClO2–NaOH–sea water system, the total removal efficiency for NOX reached 92.67%. The pH of the solution was reduced after treatment. The secret of the good performance for the series of NaClO2 samples lies in the process kinetics characteristics. The absorption process is a fast parallel reaction. The oxidation ability limits the removal effect for the NaClO oxidant series. According to the literatures [9, 10], the better functioning chemical environment for the NaClO3 oxidant series could be an acid environment, which could be a disadvantage for the chosen NaClO3 oxidant chemical environment in this work. However, these disadvantages do not hinder their potential application for the removal of NOX and SO2 from diesel engine flue gases with the combined application of EB and wet scrubber technology. At the fixed absorbed dose (10.9 kGy), the different inlet concentrations of NO for the total removal efficiency of NOX was also studied. Different oxidants (NaClO2, NaClO3) show different trends with increasing the inlet NO concentration. The ion chromatography analysis technique was applied to determine the concentration of two cations (Na+ and Ca2+) and three anions (NO3−, NO2−, and Cl−) in the post-process solutions. It was shown that with the application of EB (EB), the EB induced oxidation process produced large amounts of anions such as NO3−. With the addition of NaClO2 into the scrubber solution, the oxidation efficiency of NO to NO2 and NO2 to NO3− was further increased. The results of this study indicate that EB combined with a wet scrubbing method is very promising for application in the clean-up of SO2 and NOX emitted from the diesel engines of cargo ships. A process optimization study which will lead to lower consumption of oxidant and improve process efficiency with the application of the EB and multi-stage wet scrubber process is under development at our institute.
References
Paoletti E, Manes F (2003) Effects of elevated carbon dioxide and acidic rain on the growth of holm oak. Dev Environ Sci 3:375–389. https://doi.org/10.1016/S1474-8177(03)03021-3
Reza I, Huub HJC, Marc AD, Edward DS (2005) Literature review of air pollution control biofilters and biotrickling filters for odor and volatile organic compound removal. Environ Prog 24(3):254–267. https://doi.org/10.1002/ep.10077
Chmielewski AG, Han B (2016) Electron beam technology for environmental pollution control. Top Curr Chem 374:68. https://doi.org/10.1007/s41061-016-0069-4
Chmielewski AG, Licki J, Pawelec A, Tymiński B, Zimek Z (2004) Operational experience of the industrial plant for electron beam flue gas treatment. Radiat Phys Chem 71:439–442. https://doi.org/10.1016/j.radphyschem.2004.03.020
Chmielewski AG, Zwolińska E, Licki J, Sun YX, Zimek Z, Bułka S (2018) A hybrid plasma-chemical system for high-NOx flue gas treatment. Radiat Phys Chem 144:1–7. https://doi.org/10.1016/j.radphyschem.2017.11.005
Licki J, Pawelec A, Zimek Z, Witman-Zając S (2015) Electron beam treatment of simulated marine diesel exhaust gases. Nukleonika 60:689–695. https://doi.org/10.1515/nuka-2015-0098
Guo RT, Hao JK, Pan WG, Yu YL (2015) Liquid phase oxidation and absorption of NO from flue gas: a review. Sep Sci Technol 50:310–321. https://doi.org/10.1080/01496395.2014.956761
Monoj KM, Vaishnava RC (2013) New experimental results of combined SO2 and NO removal from simulated gas stream by NaClO as low-cost absorbent. Chem Eng J 217:48–53. https://doi.org/10.1016/j.cej.2012.12.002
Kaczur JJ, Iacoviello SA, Duncan LD (1994) Process for removal of NOX and SOX oxides from waste gases with chloric acid. United States Patent. Patent Number: 5,328,673
Kaczur JJ (1996) Oxidation chemistry of chloric acid in NOX/SOX and air toxic metal removal from gas streams. Environ Prog 15(4):245–254. https://doi.org/10.1002/ep.670150414
Acknowledgement
This work is financially supported by Polish National Center for Research and Development (Grant No. TANGO2/341079/NCBR/2017).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Zhao, L., Sun, Y., Chmielewski, A.G. et al. NO Oxidation with NaClO, NaClO2, and NaClO3 Solution Using Electron Beam and a One Stage Absorption System. Plasma Chem Plasma Process 40, 433–447 (2020). https://doi.org/10.1007/s11090-019-10022-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-10022-9