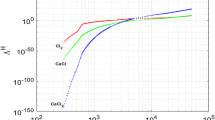
Abstract
The approach, which was developed earlier for modeling chemical reactions in laser induced plasmas, is applied to radio-frequency discharge plasmas. The model is based on the assumption that all ionization processes and chemical reactions are at local thermodynamic equilibrium. A chemical composition of an argon-hydrogen plasma with an addition of boron trichloride is studied as a function of plasma temperature and mole ratio \({\text {H}}_2/{\text {BCl}}_3\). It is established that more than twenty simple and composite molecules and ions can be formed in the course of chemical reactions. The results are compared with those obtained earlier by means of another equilibrium model that uses ab-initio quantum chemical computations of thermochemical and kinetic data and a 0D thermochemical equilibrium solver.





Similar content being viewed by others
References
Handbook of Chemistry and Physics (1971) The Chemical Rubber Co., Cleveland, OH, 52nd edn., p 72
Matkovich VI (1977) Boron and refractory borides. Springer, New York
Braganza C, Vepřec S (1979) J Nucl Mater 85–86:1133–1137
Kobayashi M, Oyama T, Nishizawa H, Ishii T, Takeuchi J (1989) Mater Sci Lett 8:403–404
Shabarova LV, Plekhovich AD, Kut’in AM, Sennikov PG, Kornev RA (2019) High Energy Chem 53:148–154
Shabanov SV, Gornushkin IB (2015) Appl Phys A 121:1087–1107
Shabanov SV, Gornushkin IB (2016) Appl Phys A 122:676
Shabanov SV, Gornushkin IB (2018) Appl Phys A 124:716
Sezgi NA, Dogu T, Ozbelge HO (1999) Chem Eng Sci 54:3297–3304
Vandenbulcke L, Vuillard G (1976) J Electrochem Soc 123:278–285
Vandenbulcke L, Vuillard G (1977) J Electrochem Soc 124:1931–1937
Sekine T, Nakanishi N, Kato E (1989) J Jpn Inst Met 53:698
Reinisch G, Vignoles GL, Leyssale J-M (2011) J Phys Chem A 115:11579–11588
Reinisch G, Leyssale J-M, Vignoles GL (2011) J Phys Chem A 115:4786–4797
Reinisch G, Leyssale J-M, Bertrand N, Chollon G, Langlais F, Vignoles G (2008) Surf Coat Technol 203:643–647
https://cantera.org/index.html. Accessed 9 Apr 2019
Fridman A (2008) Plasma chemistry. Cambridge University Press, Cambridge, p 448
Hulquist AE, Sibert ME (1969) In: Chemical reactions in electrical discharges. Advances in chemistry series, vol 80. American Chemical Society, Washington, p 182
Tzvetkov YuV, Panfilov SA (1980) Nizkotemperaturnaya plazma v protsessakh vosstanovleniya. Nauka, Moscow (in Russian)
Sennikov PG, Kornev RA, Shishkin AI (2017) Plasma Chem Plasma 37:997–1008
Casey JD, Haggerty JS (1987) J Mater Sci 22:737–744
Savastenko N, Volpp H-R, Gerlach O, Strehlau W (2008) J Nanopart Res 10:277–287
Kornev RA, Sennikov PG, Shabarova LV, Shishkin AI, Drozdova TA, Sintsov SV, Vodopyanov AV (2019) High Energy Chem 53:246–253
Tatum JB (1966) Pub Dom Ap Obs Victoria 13:1–17
Atkins P, de Paula J (2018) Physical chemistry, Ch. 17. Oxford University Press, Oxford
Irwin AW (1988) Astron Astrophys Suppl Ser 74:145–160
Drawin H-W, Felenbok P (1965) Data for plasma in local thermodynamic equilibrium. Gaunthier-Villas, Paris
Irwin AW (1981) Astrophys J Suppl Ser 45:621–633
Rienstra-Kiracofe JC, Tschumper GS, Schaefer HF (2002) Chem Rev 102:231–282
Andersen T (2004) Phys Rep 394:157–313
Popovas A, Jørgensen UG (2016) Astron Astrophys 595:A130
Huber KP, Herzberg G (1979) Molecular spectra and molecular structure. Van Nostrand Reinhold Co, New York
Simons J, Jordan KD (1987) Chem Rev 87:535–555
Xing W, Shi D, Sun J, Zhu Z (2017) Spectrochim Acta Part A 185:349–364
Hanley L, Anderson SL (1987) J Phys Chem 91:5161–5163
Bruna PJ, Write JS (1990) J Phys B At Mol Opt Phys 23:2197S–2215S
Christophorou LG, Olthoff JK (1999) J Phys Chem Ref Data 28:131–169
Peyerimhoff SD, Buenker RJ (1981) Chem Phys 57:279–296
Midda S, Das AK (2003) J Mol Struct (Theochem) 640:183–189
Miliordos E, Mavridis A (2008) J Chem Phys 128:144308
Bauschlicher CW Jr, Langhoff SR, Taylor PR (1990) J Chem Phys 93:502
Zhang Q-Q, Yang C-Lu, Wang M-S, Ma X-G, Liu W-W (2017) Spectrochim Acta Part A Mol Biomol Specrosc 182:130–135
https://cccbdb.nist.gov/elecaff1.asp. Accessed 9 Apr 2019
Irikura KK, Johnson RD III, Hudgens JW (2000) J Phys Chem A 104:3800–3805
Magoulas I, Papakondylis A, Mavridis A (2015) Int J Quant Chem 115:771–778
Hildenbrand DL (1996) J Chem Phys 105:10507
https://cccbdb.nist.gov/ie1x.asp. Accessed 9 Apr 2019
Rablen PR, Hartwig JF (1996) J Am Chem Soc 118:4648–4653
Sunahori FX, Gharaibeh M, Clouthier DJ (2015) J Chem Phys 142:174302
Rasul G, Prakash GKS, Olah GA (2000) J Phys Chem A 104:2284–2286
Nicolaides CA, Chrysos M, Valtazanos P (1990) J Phys B At Mol Opt Phys 23:791–800
Warschkow O, Lee EPF, Wright TG (1997) J Chem Soc Faraday Trans 93:53–61
Schlegel HB, Baboul AG (1996) J Phys Chem 100:9774–9779
Baeck KK, Bartlett RJ (1997) J Chem Phys 106:4604–4617
Christophorou LG, Olthoff JK (2002) J Phys Chem Ref Data 31:971–988
Gharaibeh MA, Nagarajan R, Clouthier DJ, Tarroni R (2015) J Chem Phys 142:014305
Peterson KA, Woods RC (1988) J Chem Phys 88:1074–1079
Jacox ME (2003) J Phys Chem Ref Data 32:1–441
Allendorf MD, Melius CF (1997) J Phys Chem A 101:2670–2680
Grant DJ (2010) Structure, heats of formation, and bond dissociation energies of group IIIA-group IVA molecules for chemical hydrogen storage systems. PhD Thesis, Tuscaloosa, Alabama
Galbraith JM, Vacek G, Schaefer HF III (1993) J Mol Strut 300:281–288
Acknowledgements
The authors are very grateful to Dr. K. Rurack and Prof. U. Panne for the support of this project. The authors also thank Prof. A. Kazakov for useful discussions and help in preparation of this manuscript. P.S acknowledges the RSF Grant support No 17-13-01027.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Partition Functions for Molecules and Their Ions
The partition functions for diatomic molecules and their ions used in simulations are calculated using the equation of Tatum [24]
Here \(T_e\) is the electronic state energy (\(T_0=0\) for the ground state), \(\omega _e\) is the vibration frequency, \(\omega _ex_e\) is the anharmonicity, \({\text {B}}_e\) is the rotational constant, \(\alpha _e\) is the rotation-vibration coupling, and \(\sigma\) is the symmetry number that accounts for the number of indistinguishable orientations of a molecule. The overall factor in the sum (1) is the vibrational zero-point-energy (ZPE) that is the energy difference between the vibrational ground state and the lowest point on the potential energy surface. The maximal vibrational number \(N_e\) for each electronic state is limited by the depth of the potential well is given by \(N_e=\frac{1}{2}\large \left( \frac{\omega _e}{\omega _e\chi _e}-1 \right)\).
The partition functions for tri- and four-atomic molecules and their ions are calculated using the Born-Oppenheimer approximation. The approximation assumes that the modes of molecular motion (vibrational, rotational, translational) are independent (decoupled) from each other and thus the total partition function can be factorized into a product of individual partition functions. This approach does not account for anharmonicity and, hence, permits a representation of the sums over vibrational energies in a closed form using the geometric series. For polyatomic molecules the partition function is calculated via [25, 26]
where \(U^{rot}_e(T)\) is the rotational partition function which is \(U^{rot}_e(T)=\left( \frac{kT}{hc}\right) ^{3/2}\left( \frac{\pi }{ABC}\right) ^{1/2}\) for non-linear polyatomic molecules with three rotational constants A, B, and C and the value \(U^{rot}_e(T)=\frac{kT}{chB_e}\) for linear polyatomic molecules with one rotational constant \({\text {B}}_e\). Each rotational constant is proportional to the moment of inertia of the molecule about the corresponding principal axis of the moment of inertia tensor, e.g. \(A=\frac{h}{8\pi ^2cI_A}\). Therefore, the rotational constants can easily be calculated if the moments of inertia are known or calculated from molecular geometry and spectroscopic constants. The number of vibrational frequencies is \(M_e=3M-6\) for non-linear polyatomics and \(M_e=3N-5\) for linear polyatomics with N being the number of constituent atoms. Note the rotational partition function \(U^{rot}_e(T)\) depends on an electronic state e because some molecules change their geometry (e.g. from bent to linear) depending on the electronic excitation. An example is the \({\text {BCl}}_2\) molecule which is bent in its ground electronic state and stretched (linear) in the excited electronic state.
H, \({\text {H}}^+, {\text {H}}^-\)
The partition function for H is taken from [27]. The ion \({\text {H}}^+\) has no electrons; its “electronic” partition function is therefore 1. The ground state of the anion \({\text {H}}^-\) has two electrons with two oppositely oriented spins; its degeneracy is 1. The anion has no excited states with energies below the ionization (electron affinity) energy \(I({\text {H}}^-)=0.75\,{\text {eV}}\) and therefore the full electronic partition function of \({\text {H}}^-\) is also 1.
Cl, \({\text {Cl}}^+, {\text {Cl}}^-\)
The partition functions for Cl and \({\text {Cl}}^+\) are from [27]. The partition function for the \({\text {Cl}}^-\) anion was obtained by the polynomial approximation from [28], and \(I({\text {Cl}}^-)=3.613\) eV (experimental).
B, \({\text {B}}^+, {\text {B}}^-\)
The approximation by a polynomial [28] is used to calculate the partition functions for B and \({\text {B}}^+\). The electron affinity of boron is \(I({\text {B}}^- )=0.28\,{\text {eV}}\) [29] and the ground state of \({\text {B}}^-\) is \(2s^2 2p^2~^3P\) [30]. The possible configurations for 2 equivalent \(p-\)electrons \((np^2)\) are \(^3P_2\), \(^3P_1\), and \(^3P_0\) with degeneracies 5, 3, and 1, correspondingly. With no excitations owing to the low \(I({\text {B}}^- )\) the partition function of \({\text {B}}^-\) is 9.
\({\text {H}}_2, {\text {H}}_2^+, {\text {H}}_2^-\)
The partition function for \({\text {H}}_2\) is conveniently calculated using the recent polynomial approximation [31]. For \({\text {H}}_2^+\), the electronic states \(X^2 \Sigma _g^+\), \({\text {B}}^2 \Sigma _g^+\), and \(C^2 \Pi _u\) given in [32] were used to calculate the partition function via the spectroscopic constants using Eq.1. The ground state of the anion \({\text {H}}_2^-\) is metastable; that is, there exists no bound anion state [33].
\({\text {B}}_2, {\text {B}}_2^+, {\text {B}}_2^-\)
Spectroscopic parameters for 20 first electronic states of \({\text {B}}_2\) up to \(T_e=34003~{\text {cm}}^{-1}\) are taken from [34]. The higher states are unimportant due to the dissociation energy limit \(D(B_2 )=2.71~ eV\). For \({\text {B}}_2^+\), only 3 first electronic states, \(X^2 \Sigma _g^+\), \(1^2 \Pi _u\), and \(1^4 \Pi _g\) yield the positive excitation energy \(E_e=D_0-T_e+1/2\omega _e\) for the experimental value of the dissociation energy \(D_0 (B_2^+ )=1.2\) eV [35]. For the radical \({\text {B}}_2^-\), 10 electronic states are considered listed in Table 4 in [36]. The partition functions for the three species are calculated via Eq.1.
\({\text {Cl}}_2, {\text {Cl}}_2^+, {\text {Cl}}_2^-\)
The \(X^3 \Sigma _g^+\) and \(^3\Pi _u\) states are used for the molecule \({\text {Cl}}_2\) [32]. The ionization potential and dissociation energy are \(I(Cl_2 )=11.50\) eV and \(D_0 (Cl_2 )=2.48\) eV (experiments [37]). Higher electronic states (the states \(^1P\) and higher that dissociate into the same channel as the ground state) practically have no potential well (due to a low dissociation energy of \({\text {Cl}}_2\)) [37] and can therefore be omitted. For \({\text {Cl}}_2^+\), the excited states \(X^2 \Pi _g\) and \(^2\Pi _u\) are used. The data are from [38]. For the state \(^2\Pi _u\) , Table 6 from [38] was used to obtain \(T_e\) and \(\omega _e\), the rest of the data was calculated by means of Eq.1 with an experimental value of \(R_e\) [18]. The states \(^2\Sigma _g^-\) (with \(T_e=31940~{\text {cm}}^{-1}\)) and higher appear to be irrelevant for the temperature range in which \({\text {Cl}}_2^+\) can exist. For \({\text {Cl}}_2^-\), only the ground electronic state \(X^2 \Sigma ^+\) was used. The electron affinity is \(I(Cl_2^- )=2.86\,{\text {eV}}\). All the data are from ab initio calculations [39]. The partition function for the \({\text {Cl}}^-\) anion was obtained by the polynomial approximation from [28], and \(I({\text {Cl}}^- )=3.613~ eV\) (exper.)
BH, \({\text {BH}}^+, {\text {BH}}^-\)
For BH, the data for states \(X^1 \Sigma ^+\), \(a^3 \Pi\), and \(A^1\Pi\) from Table 3 in [40] are used. Higher electronic states are unimportant as they exceed the dissociation energy \(D_0 (BH)=3.53\) eV [41]. For \({\text {BH}}^+\) and \({\text {BH}}^-\) the data for spectroscopic constants of states \(1^2 \Sigma ^+\) and \(1^2 \Pi\), correspondingly are taken from Table 2 in [42]. The higher states are irrelevant as they exceed the dissociation energy of the cation \(D_0 ({\text {BH}}^+ )=1.95\) eV [32] and ionization energy of the anion \(I({\text {BH}}^- )=0.3\,{\text {eV}}\) (exper.) [43].
\({\text {BCl}}, {\text {BCl}}^+, {\text {BCl}}^-\)
Three electronic states \(X^1 \Sigma ^+\), \(a^3 \Pi _1\), and \(A^1 \Pi\) are considered for BCl [44] and one metastable state \(X^1 \Sigma ^+\) for \({\text {BCl}}^-\) [45]; with the electron affinity being \(I({\text {BCl}}^- )=0.3\,{\text {eV}}\). The authors could not find any data in the literature about electronic states of the cationic radical \({\text {BCl}}^+\). This radical is unlikely to be present in large amount in the plasma owing to a large 2-fold difference between the dissociation and ionization energies of the BCl molecule, 5.26 eV [46] and 10.2 eV [47], correspondingly. The partition function of the \({\text {BCl}}^+\) cation is set to zero; it is excluded from calculations.
\({\text {HCl}}, {\text {HCl}}^+, {\text {HCl}}^-\)
The data for one electronic state of HCl, \(X^1 \Sigma ^+\), and two electronic states of \({\text {HCl}}^+\), \(X^2 \Pi _1\) and \(A^2 \Sigma ^+\), are from [32]. The higher states for HCl are omitted as their energies are at \(>9.5\) eV and greatly exceed the dissociation energy \(D_0 (HCl)=4.43\) eV. The molecule HCl forms no stable anion \({\text {HCl}}^-\), its electron affinity \(I({\text {HCl}}^- )=-0.614\) eV is negative [43].
\({\text {BH}}_2, {\text {BH}}_2^+, {\text {BH}}_2^-\)
The molecule \({\text {BH}}_2\) has the bent ground \(\tilde{X}^2 A_1\) and the linear \(\tilde{A}^2 B_1 (\Pi _u)\) first excited states. In the ground state, the molecule belongs to the \(C_{2v}\) symmetry group with \(\sigma =2\) the symmetry number and \(129^o\) the angle between the two B–H bonds. The ionization energies are \(I({\text {BH}}_2 )=9.8\) eV and \(I({\text {BH}}_2^-)=0.39\) eV for the neutral and anionic species, respectively [33, 47]. The full dissociation energy is \(D_0 ({\text {BH}}_2 )=7.03\) eV [48]. The data for the ground and first excited state are taken from Tables 3 and 7 in [49]. These data are enough to calculate the partition function of \({\text {BH}}_2\) via Eq.2. For the \({\text {BH}}_2^+\) cation, the linear \(D_{\infty h}\) symmetrical structure was calculated [50]. As regards the degree of stability of \({\text {BH}}_2^+\), it was found that the local minimum of the \({\text {BH}}_2^+\) ground state cannot support any vibrational levels, i.e. the molecule does not exist [51]. The \({\text {BH}}_2^+\) cation is excluded from calculations. As for the anion \({\text {BH}}_2^-\), no data is found concerning its electronic states or spectroscopic constants. It is concluded that this anion is unlikely to form and is also excluded from calculations.
\({\text {BCl}}_2, {\text {BCl}}_2^+, {\text {BCl}}_2^-\)
The molecule \({\text {BCl}}_2\) has the symmetric ground state \(\tilde{X}^2 A_1\) with spin 1/2 and degeneracy \(g_e=2\); it belongs to the \(C_{2v}\) symmetry group. The angle between the B–Cl bonds is \(125^o\), the ionization and dissociation energies are 7.33 eV and 8.61 eV, correspondingly [52]. The vibrational frequencies, 299.7, 742.1, and \(1034.5\,{\text {cm}}^{-1}\) and rotational constants, 2.84, 0.103, and \(0.100\,{\text {cm}}^{-1}\) are taken from [53]. The \({\text {BCl}}_2^+\) cation has a linear ground state \(\tilde{X}^2 \Sigma _g^+\) with the fundamental frequencies 588.4, 482.8, and \(1504.2\,{\text {cm}}^{-1}\) and the rotational constant \(0.784\,{\text {cm}}^{-1}\) [52]. The \({\text {BCl}}_2^-\) anion was investigated theoretically in [54]. The dissociation energy of \({\text {BCl}}_2^-\) into BCl and \({\text {Cl}}^-\) was calculated to be 0.52 eV. Making use of the Cl electron affinity equal to 3.613 eV and the dissociation energy of \({\text {BCl}}_2\) into BCl and Cl equal to 3.39 eV [55] the electron affinity of the \({\text {BCl}}_2\) molecule is calculated to be \(3.613+0.52-3.39=0.743\) eV. The ground state singlet \(\tilde{X}^1 A_1\) and excited state triplet \(a^3 B_2\) were predicted but the only ground state was found to be stable with the vibration frequencies 242.3, 556.6, and \(453.5\,{\text {cm}}^{-1}\). The rotational constants 1.17, 0.103, and \(0.094\,{\text {cm}}^{-1}\) are calculated from the moments of inertia using geometrical data (\(r_{{\mathrm{B}}-{\mathrm{Cl}}}=1.962~\AA\) and \(\angle ({\mathrm{Cl}}-{\mathrm{B}}-{\mathrm{Cl}})=101.7^o\)) . These data are used to calculate the partition function by means of Eq.2.
BHCl, \({\text {BHCl}}^+, {\text {BHCl}}^-\)
The molecule BHCl belongs to the \(C_S\) symmetry group in its ground state \(X^2 \tilde{A}_1\) with the angle between H–B and B–Cl bonds \(\angle ({\text {H}}-{\text {B}}-{\text {Cl}})=123.3^o\) and the bond lengths \(r_{H-B}=1.19~\AA\) and \(r_{{\mathrm{B}}-{\mathrm{Cl}}}=1.72~\AA\) [56]. The symmetry number is \(\sigma =1\). The vibration frequencies are 842, 909, and \(2641\,{\text {cm}}^{-1}\), the rotational constants 20.003, 0.5850, and \(0.5683\,{\text {cm}}^{-1}\) are calculated from the geometrical data. The molecule has also a linear excited state \(A^2 A^{''}\Pi ~ (\sigma =2, g_e=2)\) with \(T_e=6208\,{\text {cm}}^{-1}\), frequencies 2861, 926, and \(682\,{\text {cm}}^{-1}\), and rotational constant \({\text {B}}_e=0.547\,{\text {cm}}^{-1}\). A cation of BHCl has two forms: \({\text {BHCl}}^+\) and \({\text {BClH}}^+\); at the MP2 level of approximation the minimum of the linear \({\text {BHCl}}^+\) lies 4.1 eV below the MP2 minimum of \({\text {BClH}}^+\) [57], therefore only the \({\text {BHCl}}^+\) cation is considered. The linear \({\text {BHCl}}^+\) molecule has the \(\tilde{X}^1\Sigma ^+\) ground state [58] with the vibrational constants 2876.3, 716.1, and \(1134.1\,{\text {cm}}^{-1}\) and the rotational constant \({\text {B}}_e=0.629\,{\text {cm}}^{-1}\). No data are found for the anion \({\text {BHCl}}^-\); it is further assumed that the BHCl does not have a stable anion and its electron affinity is taken zero.
\({\text {BH}}_2{\text {Cl}}, {\text {BH}}_2{\text {Cl}}^+, {\text {BHCl}}^-\)
The molecule \({\text {BH}}_2{\text {Cl}}\) has a \(\tilde{X}^1 A_1\) ground state with the \(C_{2v}\) symmetry (\(\sigma =2\)), 6 vibrational frequencies 868.5, 919.7, 1044.2, 1291.2, 2709.7, \(2826.4\,{\text {cm}}^{-1}\) and 3 rotational constants 7.65, 0.527, and \(0.493\,{\text {cm}}^{-1}\) [53]. The full dissociation energy is 12.35 eV [59]. The cation \({\text {BH}}_2 {\text {Cl}}^+\) is unlikely to form because the ionization energy of \({\text {BH}}_2 {\text {Cl}}\), which is assumed to be similar to that of the \({\text {BHCl}}_2\), i.e. 12 eV, are much higher than the dissociation energy 5.36 eV of \({\text {BH}}_2 {\text {Cl}}\) into \({\text {BH}}_2\) and Cl and the dissociation energy 4.49 eV of \({\text {BH}}_2 {\text {Cl}}\) into BHCl and H [60]. Adding an electron changes the \({\text {BH}}_2 {\text {Cl}}\) symmetry from planar \(C_{2v}\) to pyramidal \(C_s\). So, the \({\text {BH}}_2 {\text {Cl}}^-\) anion is an asymmetric top molecule (\(\sigma =1\)). The electron affinity of \({\text {BH}}_2 Cl\) is only 0.05 eV [60]; the anion is therefore neglected.
\({\text {BHCl}}_2, {\text {BHCl}}_2^+, {\text {BHCl}}_2^-\)
The molecule \({\text {BHCl}}_2\) is of the \(C_{2v}\) symmetry in its ground state \(\tilde{X}^1 A_1\) with vibrational frequencies 308.6, 772.8, 824.3, 953.7, 1157.2, \(2792.8\,{\text {cm}}^{-1}\) and rotational constants 1.60, 0.106, and \(0.100\,{\text {cm}}^{-1}\) [53]. The dissociation and ionization energies are 13.12 eV [47] and 11.91 eV [59], respectively. The cation \({\text {BHCl}}_2^+\) is not considered; it is unlikely to be created because its ionization energy 11.91 eV is more than 2-fold higher than the dissociation energy 5.29 eV of \({\text {BHCl}}_2\) into BHCl and Cl and the dissociation energy 4.48 eV of \({\text {BHCl}}_2\) into \({\text {BCl}}_2\) and H [60]. Similar to \({\text {BH}}_2 {\text {Cl}}^-\), the \({\text {BHCl}}_2^-\) anion has the \(C_s\) symmetry. The electron affinity of \({\text {BH}}_2 {\text {Cl}}\) is fairly low, 0.22 eV [60]; this anion is also neglected.
\({\text {BH}}_3, {\text {BH}}_3^+, {\text {BH}}_3^-\)
A borane molecule \({\text {BH}}_3\) has the full dissociation energy \(D_0 ({\text {BH}}_3 )=11.51\) eV [48], ionization energy 12.03 eV [47], and electron affinity 0.038 eV [29]. This is a trigonal planar molecule with the \(D_{3h}\) symmetry (\(\sigma =6\)) in the ground state \(\tilde{X}^1 A_1^{'}\) [61]. The vibrational frequencies 1191.8, 1255.1, 1255.1, 2646.9, 2789.8, \(2789.8\,{\text {cm}}^{-1}\) and the rotational constants 7.86, 7.86, and \(3.93\,{\text {cm}}^{-1}\) are from [53]. The \({\text {BH}}_3^+\) cation is unimportant because the dissociation energy 4.62 eV of \({\text {BH}}_3\) into \({\text {BH}}_2\) and H is much lower than its ionization energy. The anion \({\text {BH}}_3^-\) is also unimportant due to the very low electron affinity of the \({\text {BH}}_3\).
\({\text {BCl}}_3, {\text {BCl}}_3^+, {\text {BCl}}_3^-\)
A boron trichloride molecule \({\text {BCl}}_3\) is a trigonal planar molecule with the \(D_{3h}\) symmetry (\(\sigma =6\)) in the ground state \(\tilde{X}^1 A_1^1\) [54]. The dissociation and ionization energies and electron affinity are 13.77 eV, 11.64 eV, and 0.33 eV, respectively [55]. The vibrational frequencies 272.6, 272.6, 479.9, 496.9, 1009.1, \(1009.1\,{\text {cm}}^{-1}\) and rotational constants 0.107, 0.107, and \(0.053\,{\text {cm}}^{-1}\) are from [53]. The cation \({\text {BCl}}_3^+\) is unimportant as the dissociation energy 4.61 eV of \({\text {BCl}}_3\) [55] is much smaller than the ionization energy. The anion \({\text {BCl}}_3^-\) has \(C_{3v}\) symmetry, its ground state is \(\tilde{X}^2 A_1^1\) [54]. The vibrational frequencies 551, 315, 695, 695, 214, \(214\,{\text {cm}}^{-1}\) are from [48] and rotational constants 0.101, 0.101, and \(0.052\,{\text {cm}}^{-1}\) are calculated based on the geometrical data in [54]].
Rights and permissions
About this article
Cite this article
Gornushkin, I.B., Shabanov, S.V. & Sennikov, P.G. Equilibrium Chemistry in \({\text {BCl}}_3\)–\({\text {H}}_2\)–Ar Plasma. Plasma Chem Plasma Process 39, 1087–1102 (2019). https://doi.org/10.1007/s11090-019-09985-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11090-019-09985-6