Abstract
This study provides a comprehensive investigation of the formation and behavior of a layer enriched in fission products encountered between the (U,Pu)O2 fuel pellet and the cladding, designated as JOG (“Joint Oxyde Gaine” in French). Employing a multifaceted approach that combined thermodynamic calculations, experimental synthesis, and advanced characterization techniques, simulated JOG has been synthetized (without radioactive fission products). Using thermodynamic calculations with the TAF-ID database, the phase compositions in the JOG was assessed for various temperatures, pressures, and oxygen potential conditions, revealing insights into the environmental factors influencing JOG formation. Experimental simulation of the JOG composition, exposed to controlled conditions, confirmed the presence of key compounds such as Cs2MoO4, CsI, and PdTe, as evidenced by SEM, EDS, and XRD analyses. The results of the calculations highlighted notable differences in the nature of the phases constituting the JOG under varying pressure and oxygen potential conditions. At 873 K and oxygen partial pressure of 10–4 bar, Cs2MoO4, Pd–Te, and a gas phase rich in tellurium and CsI were predominant, contrasting with the emergence of liquid phases at 70 bar. This study offered a comprehensive understanding of JOG microstructure, and highlighting the importance of accurate characterization for reactor safety. This information lays the foundations for future studies on the chemical interaction between the JOG and the steel cladding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
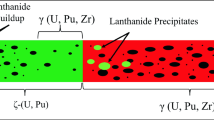
In the global effort to tackle energy and environmental challenges, there is a considerable focus on advanced nuclear technologies, notably sodium-cooled fast reactors (SFRs). These efforts are crucial for achieving ambitious goals of reducing greenhouse gas emissions while ensuring a safe and sustainable energy supply [1]. The commercialization of SFRs holds great promise for the future of nuclear energy. Specifically, SFRs using mixed oxide (U,Pu)O2 (MOX) fuels offer high burnup rates exceeding 20% fissions per initial metal atom (FIMA), demonstrating the ability to operate at high burnup rates [2]. However, for SFRs to become economically competitive with current light water reactors, fuel assemblies must achieve even higher burnup beyond 15% FIMA [3]. Unfortunately, the operating conditions of SFRs, characterized by high temperatures, lead to significant migration of fission products, resulting in fuel-cladding chemical interaction (FCCI) [4]. In fact, the large thermal gradient between the center and periphery of the (U,Pu)O2 (MOX) pellet induces the migration of the most volatile fissions products (Cs, I, Te, Mo, Ba, Pd, O) from the center (T ~ 2273 K) toward the periphery (T ~ 873 K). This migration leads to the accumulation of these fission products in the gap between the fuel pellet’s periphery and the steel cladding, resulting in the formation of a layer enriched in fission products designated as JOG (“Joint Oxyde Gaine” in French) [5, 6]. The fission products of the JOG can then attack the steel cladding leading to its corrosion. This chemical interaction is a major factor limiting the lifetime of SFR fuel pins in the reactor [7].
Up to now, data on the structure and accurate chemical composition of the JOG are scarce [8]. The JOG represents a complex chemical system involving many elements such as Cs, Mo, Te, Pd, Ba, I, and O, stabilized in multiple compounds like Cs2MoO4, CsI, and Cs2Te according to thermodynamic calculations [9]. So far, only a few compounds have been identified in the JOG, notably Cs2MoO4, which has been established as the main phase in various studies including post-irradiation examination (PIE) analyses [10], electron probe microanalyses (EPMA) [5], and thermodynamic calculations [9, 11, 12]. Nevertheless, the literature also suggests the presence of other fission products such as Pd, Te, and Ba [5, 13, 14]. Despite this progress, the remaining phases of JOG are still not clearly identified, highlighting the need for in-depth research to improve our understanding of its structure and chemical composition.
This study aims to provide a comprehensive understanding of the JOG composition (formed phases and their chemical nature) by employing a multi-faceted approach. Thermodynamic calculations with the TAF-ID-V16 database [15] were performed to evaluate the phases composition and mole fraction in the JOG as a function of temperature and oxygen chemical potential under various pressures. This computational analysis provides a global understanding of how various environmental factors influence the formation and stability of the JOG within nuclear reactor environments. In addition, a simulated JOG was synthesized by mixing various fission product compounds commercially available, including Cs2MoO4, BaMoO4, Te, Pd, and CsI. Chemical and structural characterizations were then conducted using scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), and XRD analyses.
Experimental Procedures
The composition of the real JOG observed in the NESTOR3 irradiation campaign was estimated using calculations with the GERMINAL fuel performance code [16]. JOG-NESTOR3 consists of Cs, Te, I, Mo, O, Ba and Pd (elemental composition is given in Table 1). Thermodynamic calculations based on the elemental composition of JOG- NESTOR3 led to the compounds listed in Table 1. The simulated JOG called JOG-1 was synthesized by mixing powders of several compounds (Cs2MoO4, BaMoO4, Te, Pd, and CsI) with the aim of coming as close as possible to NESTOR3’s JOG composition [16]. The mass compositions of the sample consisting of 56% Cs2MoO4, 31% Te, 10% CsI, and 3% Pd, are detailed in Table 1. But the compounds Cs2O and Cs2Te are not commercially available. As a result, the Cs content of JOG-1 is lower than that of JOG-NESTOR3; while, the oxygen content is higher (with reference to the percentage values in bold for Cs and O in Table 1). To maintain an adequate proportion of Cs and Te in JOG-1, it was decided to use larger quantities of Cs2MoO4 and Te.
The manufacturing method involves manually mixing and pressing of the powders to form a pellet with a diameter of 7 mm. This JOG-1 pellet was then placed into an alumina crucible and heated at 873 K for 4 h into a quartz ampoule sealed under primary vacuum (~ 10–4 bar). It should be noted that all these experimental steps, from synthesis to sample heating and metallurgical preparation, were conducted in a glove box under argon atmosphere to prevent reactions with air and/or water vapor.
Results and Discussion
Thermodynamic calculations were performed considering two pressure conditions: 10–4 bar representing the synthesis condition in a sealed ampoule for our simulated JOG-1 and 70 bar, the maximum internal pressure found in the fuel pin NESTOR3 (JOG-NESTOR 3) [17]. This comparative analysis aims to explore the influence of pressure on the JOG compound formation. Figure 1 illustrates the evolution of phase molar fractions as a function of temperature for the two pressures, calculated using Thermo-Calc software and the version 17 of the TAF-ID database [13].
Calculated mole fraction of the phases using the TAF-ID [13] database in JOG-1 and JOG-NESTOR3 as a function of temperature at PTOT = 70 bar (a) and (c) and PTOT = 10–4 bar (b) and (d)
Calculations on JOG-1 under 10–4 bar (Fig. 1a) show that the solid compounds Te, Cs2MoO4, PdTe2, and CsI are stable up to 640 K. Beyond this temperature, a liquid phase forms mainly enriched in tellurium, CsI and some Cs2MoO4. From 722 K, the tellurium and CsI from this liquid phase go into the gas phase. The PdTe2 compound remains stable up to 870 K, where it undergoes a transition to the PdTe2-β phase that melts at 950 K. The α/β transition of Cs2MoO4 occurs at 841 K, which is consistent with literature data [11, 18]. β-Cs2MoO4 remains stable up to at least 1000 K.
At 70 bar (Fig. 1b), the same compounds remain stable up to 640 K. PdTe2 remains stable up to 1000 K. Beyond 640 K, a liquid phase forms, containing Te, Cs2MoO4, and CsI, whose fraction increases with temperature whereas the fraction of solid Cs2MoO4 decreases. Above 970 K, a second liquid phase forms. MoO2 and MoTe2 are found to exist with a very small fraction. No gas phase is predicted to form.
Calculations performed at 10–4 bar (Fig. 1c) on the JOG-NESTOR3 show the presence of the solid compounds Cs2MoO4, Cs2Te, CsI and FCC(Pd) in equilibrium with a liquid phase (Cs2O,TeO2) up to 690 K. Above this temperature, another liquid phase is formed, composed of Cs2O, CsI, TeO2, and Cs2MoO4, in equilibrium with the compounds Cs2Te, Pd and Cs2MoO4. As in JOG-1 (Fig. 1a), calculations indicate the formation of a gas phase from 722 K, mainly enriched in Cs and CsI. Te remains associated with Cs in the form of Cs2Te. Above 920 K, JOG-NESTOR3 is characterized by the coexistence of a gas phase rich in Cs and CsI, and a liquid phase containing Cs2Te, Cs2MoO4 and Pd.
Calculations on JOG-NESTOR 3 conducted at 70 bar (Fig. 1d) reveal the presence of the solid compounds Cs2MoO4, Cs2Te, CsI, and Pd that are stable up to 700 K, together with a liquid phase predominantly composed of Cs2O and TeO2. Above 700 K, a substantial fraction of these compounds exists in the liquid state. Importantly, no vapor phase is predicted to form in JOG-NESTOR3 at the internal pressure characteristic of the NESTOR 3 needle.
At the cladding temperature (873 K), the predicted phases differ depending on the pressure.
-
At 70 bar:
-
JOG-1 contains Cs2MoO4, PdTe2, and a liquid phase rich in Te, Cs2MoO4, and CsI;
-
JOG-NESTOR 3 is constituted of a liquid phase (Cs2Te, Cs2O, CsI, Cs2MoO4, and Cs) in equilibrium with solid Pd.
-
-
At 10–4 bar:
-
JOG-1 contains Cs2MoO4, PdTe2, and a gas phase (Te and CsI);
-
JOG-NESTOR 3 is constituted of solid Pd, Cs2Te, Cs2MoO4, and a gas phase rich in Cs and CsI.
-
These differences in results between JOG-1 and JOG-NESTOR 3 are probably due to the lower molar fraction of Cs in JOG-1. However, for both JOG compositions, as expected, the coexistence of gas and liquid phases is expected at 10–4 bar; while, there is no gas phase at 70 bar where the liquid fraction is higher. The internal pressure is thus an important factor to take into account when studying the formation mechanisms and microstructure of the JOG.
The JOG chemistry also depends on the oxygen chemical potential, as indicated in the literature [19,20,21]. Figure 2 shows the mole fraction of the phases calculated at 873 K (temperature of the cladding) as a function of oxygen potential with Thermo-Calc and the TAF-ID database for JOG-1 and JOG-NESTOR 3.
Calculated mole fraction of the phases using the TAF-ID [13] database in JOG-1 and JOG-NESTOR3 as a function of oxygen potential at 873 K at PTOT = 70 bar (a) and (c) and PTOT = 10–4 bar (b) and (d)
For JOG-1:
-
At 10–4 bar (Fig. 2a):
-
For μO2 <—380 kJ/mol, the most stable compounds are: MoTe2, Mo3Te4, and liquid (CsI-Te);
-
Beyond μO2 =—380 kJ/mol, the phases are: Cs2MoO4 (30% mol), PdTe2 (20% mol), and a vapor phase mainly composed of Te and CsI (50% mol).
-
-
At 70 bar (Fig. 2b):
-
For μO2 <—350 kJ/mol, MoTe2 and Mo3Te4 are the major phases in equilibrium with Cs2MoO4 and PdTe2, transitioning to a liquid phase below μO2 =—400 kJ/mol;
-
Beyond μO2 =—350 kJ/mol, the most stable phases are: Cs2MoO4 (10% mol), Pd (20% mol), and 70% mol. of a liquid phase containing Te, Cs2MoO4, and CsI.
-
For JOG NESTOR 3:
-
At 10–4 bar (Fig. 2c):
-
For μO2 <—500 kJ/mol, Cs2Te, Pd, Mo, and the gas phase (Cs and I) are the most stable phases;
-
Between -500 and -275 kJ/mol, stable phases include Cs2MoO4 (25% mol), Cs2Te (35% mol), PdTe (Pd rich FCC solid phase, 5% mol), and the gas phase (Cs and I);
-
Beyond µO2 ≈—275 kJ/mol: a liquid 1 forms, composed of Cs2O and TeO2 (75% mol.), a liquid 2 enriched in CsI and Cs2MoO4 (20% mol.), as well as the compound Cs2MoO4 (5% mol.).
-
-
At 70 bar (Fig. 2d):
-
liquid is the main phase predicted to form, containing primarily over 90% mol of cesium-based species (Cs2Te, Cs, Cs2O, CsI, Cs2MoO4);
-
Beyond µO2 ≈—290 kJ/mol, Cs2MoO4 remains stable; while, Mo appears in metallic form only at low oxygen potentials, typically below µO2 ≈—600 kJ/mol, together with Pd.
-
This comparison between the compositions of JOG-1 and JOG-NESTOR3 demonstrates the absence of the gaseous phase at 873 K for a pressure of 70 bar. Therefore, the most probable state expected in JOG-1 at 873 K, under an oxygen potential of − 350 kJ/mol and at the internal needle pressure [22], is defined by the presence of the Pd–Te phase, Cs2MoO4, and the liquid phase containing Te, CsI, Cs2MoO4. In all cases, because of a low pressure in the sealed ampoules, the formation of a gas phase is expected in our experiments, which is probably not the case in the fuel pin at 70 bar. However, these experiments can still provide interesting information on the JOG composition and microstructure. Comparing these calculations with our experimental results will also test the validity of these calculations.
In fact, in the present work, the chemical interactions between the fission product compounds (Cs2MoO4, CsI, Te, and Pd) are experimentally studied through the heat treatment at 873 K. Scanning electron microscope examination of the morphology of the JOG-1 pellet after heat treatment reveals a rough surface in relief, characterized by the presence of small nodular precipitates uniformly distributed over its surface. Moreover, several distinct deposits have been identified in different locations:
-
A black deposit within the alumina crucible (Fig. 3) and deposits observed on the inner surface of the quartz ampoule probably resulting from vaporization/condensation phenomena;
-
The formation and deposition of a white phase in the cold regions of the quartz ampoule.
According to the calculations conducted for the JOG-1 at 10–4 bar, the gray deposit on the quartz ampoule may correspond to Cs-Te gas phases; while, the white deposit is more likely formed from CsI(g) condensation.
X-ray diffraction analysis of the JOG-1 pellet after heat treatment (see Fig. 4) reveals the predominant presence of Cs2MoO4-α and Cs2(MoO4)-β phases. Additionally, CsI was detected. A low-intensity peak is also visible around 2θ = 33.5° and 2θ = 80°, corresponding to the compound PdTe.
Figure 5 depicts the microstructure of the JOG-1 pellet surface, as observed by SEM. In agreement with the XRD analysis of Fig. 4, the microstructure reveals the presence of nodules ranging from 20 to 100 µm diameter, likely corresponding to Cs2MoO4. These nodules are surrounded by the CsI phase, which was likely in a liquid state, as indicated by thermodynamic calculation (Fig. 1a), the pellet stuck to the crucible and SEM observations showing a smooth liquid CsI phase wetting th Cs2MoO4 nodules. Darker precipitates correspond to regions enriched in Te and Pd. After heat treatment, no Te-containing compounds other than PdTe were detected, and no pure tellurium was identified, even though the molar amount of Te before heat treatment was 10 times that of Pd (Table 1). Consequently, a significant amount of Te must have volatilized. According to Fig. 1a, at 873 K the formed phases shall be: solid Cs2MoO4, PdTe2, and a gas phase (Te, CsI). Since the amount of Te in the gas phase is 42 mol. % (calculations in Fig. 1.a), the gray deposit observed on the alumina crucible in Fig. 3 is probably mainly Te. Other X-ray mapping of the JOG-1 surface (Fig. 5) highlights the presence of liquid CsI around the Cs2MoO4 nodules. The numerous gray/black spots observed in the images correspond to the formation of PdTe.
As shown in SEM images in Fig. 6, the JOG-1 sample has a significant amount of porosities and cracks, that could be attributed to vaporization leading to the formation of Cs-Te and CsI deposits observed in Fig. 3. Figure 6 shows the presence of two phases containing Cs-Mo-O and having the same stoichiometry, according to EDX spots 1 and 2. These two different phases could be Cs2MoO4-α and Cs2MoO4-β, which were identified on the X-ray diffractogram in Fig. 4. Indeed, the allotropic transformation occurs at 841 K according to Fig. 1. At various locations, PdTe, visible in light gray at points 3 and 6, confirms the X-ray elemental mapping and the X-ray diffractogram of Fig. 4. Furthermore, a light gray region rich in Cs and I, corresponding to CsI, was detected at spot 4. The low amount of CsI phase in the JOG1 sample, despite it being the main compound after Te (see Table 1), can be explained by the calculations in Figs.1 and 2, which show CsI as a gas rather than a solid or liquid. Consequently, the CsI could vaporize and escape from the solid JOG1 sample, leading to the formation of the large observed porosities in Fig. 6a.
These observations are consistent with the results of calculations on JOG-1 performed under a pressure of 10–4 bar.
The calculations on JOG NESTOR 3 show the formation of Cs2MoO4, Cs2Te and the gaseous phase composed of Cs and CsI under the same experimental conditions. The only difference compared to JOG-1 comes from the Cs2Te phase and Pd predicted to form. The increase in the concentration ratio of Cs to Pd in JOG NESTOR 3 compared to JOG-1 induces the formation of Cs2Te (in JOG NESTOR 3) instead of PdTe2 (in JOG-1).
Conclusions
The characterization of a simulated JOG sample heated for 4 h at 873 K using SEM, EDS, and XRD analyses has confirmed the formation of Cs2MoO4, CsI, and PdTe phases. These findings deepen our understanding of phases formation in JOG in nuclear reactor environments. Furthermore, this study proves the presence of liquid (CsI) within the JOG. The presence of a liquid phase will be of great importance for the fuel-cladding chemical interaction (FCCI). Moreover, this study shows good agreement between thermodynamic calculations using the TAF-ID-V17 and experimental tests on simulated JOG. Other JOG simulation experiments will be carried out with a composition closer to that of NESTOR3 in order to gain a better understanding of the JOG microstructure. In a next step, corrosion tests between JOG samples and steel cladding will be conducted to better understand and model this corrosion phenomenon in fast reactors.
Data Availability
No datasets were generated or analyzed during the current study.
References
L. C. Walters, D. L. Porter, and D. C. Crawford, Progress in Nuclear Energy 40, 2002 (513).
M. Teague, B. Gorman, J. King, D. Porter, and S. Hayes, Journal of Nuclear Materials 441, 2013 (267).
R. D. Leggett and L. C. Walters, Journal of Nuclear Materials 204, 1993 (23).
K. Maeda, Ceramic Fuel–Cladding Interaction. In: eds. (Elsevier, Amsterdam 2012), p. 443–483.
M. Tourasse, M. Boidron, and B. Pasquet, Journal of Nuclear Materials 188, 1992 (49).
K. Maeda and T. Asaga, Journal of Nuclear Materials 327, 2004 (1).
C. Guéneau, J.-C. Dumas, and M. H. A. Piro, 11-In-reactor behavior. in Woodhead Publishing Series in Energy, ed. M. H. A. Piro (Woodhead Publishing, 2020), p. 419.
Y. Guerin, 2.21-Fuel Performance of Fast Spectrum Oxide Fuel. In: RJM Konings, eds. (Oxford, Elsevier 2012), p. 547.
J-C. Dumas, PhD thesis. Étude des conditions de formation du joint oxyde-gaine dans les combustibles des réacteurs à neutrons rapides : observations et proposition d’un modèle de comportement des produits de fission volatils. Institut National polytechnique, Grenoble, France. (1995).
F. Cappia, B. D. Miller, J. A. Aguiar, L. He, D. J. Murray, B. J. Frickey, J. D. Stanek, and J. M. Harp, Journal of Nuclear Materials 531, 2020 151964.
R. J. M. Konings and E. H. P. Cordfunke, Thermochimica Acta 124, 1988 (157).
Pham thi T ngoc. PhD thesis. Caractérisation et modélisation du comportement thermodynamique du combustible RNR-Na sous irradiation. (Aix-Marseille, France 2014)
K. Maeda, K. Tanaka, T. Asaga, and H. Furuya, Journal of Nuclear Materials 344, 2005 (274).
CEA SACLAY. Sodium cooled nuclear reactors. In: eds. (Lemoniteur, Paris, 2016)
C. Guéneau et al. Calphad 72, 102212 (2021) https://www.oecd-nea.org/jcms/pl_24703/thermodynamics-of-advanced-fuels-international-database-taf-id.
J-C. Dumas, M. Lainet, K. Samuelsson, B. Sundman, Modeling volatile fission products transport into the Germinal V2 fuel performance code by coupling to thermochemical software. In The NUMAT2018 Conference. (2018)
B. Boer, J. Eysermans, S. Lemehov, S. Cen, L. Luzzi, T. Barani, L. Cognini, A. Magni, D. Pizzocri, Results of the benchmark between pre- and post-INSPYRE code versions on selected experimental cases, 1, (2021).
H. R. Hoekstra, Inorganic and Nuclear Chemistry Letters 9, 1973 (1291).
T. B. Lindemer, T. M. Besmann, and C. E. Johnson, Journal of Nuclear Materials 100, 1981 (178).
M. G. Adamson, E. A. Aitken, and T. B. Lindemer, Journal of Nuclear Materials 130, 1985 (375).
H. Kleykamp, Journal of Nuclear Materials 206, 1993 (82).
L. Martinelli, Etude bibliographique de la corrosion interne des gaines (ROG-RIFF). CEA REPORT DPC/SCCME 12-A 2012.
Funding
Open access funding provided by Commissariat à l'Énergie Atomique et aux Énergies Alternatives.
Author information
Authors and Affiliations
Contributions
M.O., C.G., and L.M. conceived the idea presented in this paper. M.O. wrote the manuscript with in consultation with C.G. and L.M. M.O. and C.G. designed the database model for simulations. M.O. performed thermodynamic calculations, which were verified by C.G. and L.M. All three contributed to interpreting the results. M.O., K.G., C.G., and L.M. conceived and planned the experiments. M.O. and K.G. conducted the experimental synthesis and advanced characterization using SEM and EDS. R.G. performed XRD analyses. All authors provided critical feedback and contributed to shaping the research, analysis, and manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Oulfarsi, M., Guéneau, C., Ginestar, K. et al. Understanding and Predicting the Thermodynamic Behavior of Fission Products Encountered Between the (U,Pu)O2 Fuel Pellet and the Cladding: Characterization and Modeling Approaches. High Temperature Corrosion of mater. 101, 911–922 (2024). https://doi.org/10.1007/s11085-024-10266-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-024-10266-7