Abstract
Ni-based superalloys are commonly used in gas turbines because of their exceptional high-temperature mechanical properties. To secure a long service life, the materials must also have sufficient corrosion resistance. Therefore, diffusion coatings are widely used to enrich the surface in protective oxide scale-forming elements. For temperatures between 650 and 950 °C, where hot corrosion occurs, Cr-based coatings are advantageous. These are commonly applied via the laborious pack cementation process. Recently, a novel cost-effective Cr/Si slurry coating process has been developed which demonstrated resistance to oxidative high-temperature environments. Here, the protection of the slurry coatings against hot corrosion type I at 900 °C on the Ni-based superalloy Rene 80 is investigated and compared to coatings produced by pack cementation. Prior to the 300-h exposures in air containing 0.1% SO2 at 900 °C, 4 mg/cm2 of Na2SO4 was deposited on the material surfaces. The uncoated Rene 80 exhibited rapid dissolution of the initial oxide scale followed by catastrophic break away oxidation. In comparison, the slurry coatings showed significantly improved hot corrosion resistance compared to the uncoated alloy and a better protection than a Cr pack cementation coating. The Cr pack cemented Rene 80 showed improved hot corrosion resistance, but Cr depletion in the subsurface zone occurred with increasing exposure time, associated with the propagation of Al internal oxidation and increasing sulfidation. In contrast, the slurry coatings formed an external Cr2O3 scale coupled with an agglomeration of SiO2 underneath and a continuous Al2O3 subscale which offered a better diffusion barrier and leading to superior long-term protection against hot corrosion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ni-based superalloys are widely used as materials for hot sections of gas turbines. As the materials are applied in a combustion environment and therefore exposed to hot and aggressive gases, high-temperature corrosion and oxidation resistance is required. At sufficient partial pressure of SO2/SO3, components can degrade due to hot corrosion. Hot corrosion is induced by the deposition of thin salt films, most commonly sodium sulfate (Na2SO4) [1]. Deposition of Na2SO4 can occur from the reaction of NaCl from seawater with sulfur oxides from sulfur-containing fuels [2] or air pollution. Na2SO4 melts at lower temperatures (Tm = 884 °C [3]) and can then react with the oxides of the material to dissolve via an acid–base reaction [4]. Hot corrosion of Ni-based alloys by (Na2SO4) can be divided into two regimes: type II at temperatures of 500–700 °C where the salt forms low melting eutectics (i.e., Na2SO4–NiSO4 at 671 °C) and type I at 800–950 °C, where Na2SO4 melts directly and can dissolve the oxide scales without an incubation period [5]. With increasing temperature, the PSO3 decreases in favor of PSO2, shifting the salt activity from Na2SO4 to Na2O [5]. As a result, a basic dissolution becomes more likely, especially when sulfur and oxygen are also consumed by the corrosion reactions. Hot corrosion attack can be mitigated by protective oxide scales which exhibit a low solubility in Na2SO4 [6]. In general, the presence of a liquid Na2SO4 deposit shortens the time during which protective scales can form by selective oxidation due to continuous depletion, thus accelerating the corrosion attack [7, 8]. To counteract this and create a sufficient reservoir to form protective scales (of Al, Cr and/or Si), diffusion coatings are commonly applied [9, 10]. Cr-modified Al coatings or Cr-based coatings favor thermodynamically highly stable chromium sulfides, binding sulfur and preventing inward diffusion [6, 11]. Furthermore, Cr2O3 exhibits advantageous behavior by limiting the basicity of the melt when dissolved in Na2SO4. In contrast to other oxides, it has lower solubility at lower oxygen partial pressures. Thus, it reprecipitates at the oxide/metal interface and slows down the corrosion process [12].
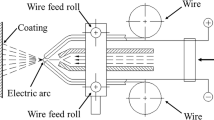
The state-of-the-art and industrially used process for chromizing is pack cementation [2]. In pack cementation the coating deposition occurs via a CVD process. The components are usually fully embedded in a powder mixture which contains the metallic source, filler material (usually Al2O3) and a halogenide activator. During the heat treatment, the halogenide reacts with the metallic element from the source and transports it to the substrate surface via the gas phase where the metal source diffuses into the material [9, 13, 14]. However, such a coating process presents limitation in costs as it requires a large quantity of powders to embed the components. An alternative and simpler process is a slurry coating, which has been already well established and widely used for Al-based coatings [15, 16]. The metal powder is mixed with a binder liquid into a slurry which is deposited on the substrate surface. During the post-heat treatment, the coating forms via interdiffusion between the powder particles and the substrate surface. The existence of a liquid phase is necessary to reach sufficient diffusion rates and form a thick enough diffusion zone [9], reducing time and cost. Because of the melting point of Cr above 1900 °C, the development of a comparable slurry process to Al-based coatings for Cr-based coatings has been challenging.
A new and environmentally friendly process to apply Cr/Si-based slurry coatings has recently been patented, and demonstrated [17, 18]. Under air at 900 °C, the coatings with high Cr contents up to a depth of 150 µm improved the oxidation resistance of Inconel 740H and Rene 80, due to the combination of a Si-rich subscale underneath a slow-growing Cr2O3 scale. Here we performed a systematic study of the hot corrosion behavior of uncoated, Cr pack cemented and Cr/Si slurry-coated Rene 80 to reveal and highlight the protective effects from the coatings.
Experimental Procedures
The Cr/Si slurry coatings and pack cementation coatings were applied on the Ni-based superalloy Rene 80 [19]. The nominal composition of Rene 80 is given in Table 1. Samples were cut into a planar geometry of (20 × 10 × 2) mm using electrodischarge machining (EDM). The procedure of the Cr/Si slurry coating was briefly aforementioned above and is described in detail in [18]. Prior to coating, both pack cementation and slurry-coated samples were sand-blasted and cleaned in an ethanol ultrasonic bath. For pack cementation, the samples were embedded into a powder mixture inside an Al2O3 crucible. The powder contained 5 wt.-% Cr powder as the metallic source, 2 wt.% MnCl2 powder as activator and 93 wt.% Al2O3 powder as filler material. The heat treatment was carried out at 1050 °C for 4 h at a gas flow of 5 l/h of Ar-5vol.% H2 in a quartz tube furnace. After coating application, all samples were ground to P1200 to remove any remaining uneven surface residue prior to the exposure tests. Before the Na2SO4 deposition, Na2SO4 powder (≥ 99% granular, Alfa Aesar, Haverhill, MA, USA) was dissolved in distilled water and the solution sprayed by an airbrush to distribute the salt evenly. During the deposition, the samples were placed on a heating plate at ~ 80 °C. A total amount of ~ 4 mg/cm2 of salt was deposited and monitored by weighing of the samples.
The quasi-isothermal (24, 100, 300 h) exposures were carried out in a 20-cm-diameter quartz tube furnace at 900 °C under a calibrated gas flow of 75 ml/min of 0.9 vol. % synthetic air and 0.1 vol.-% SO2. After each time step, a sample of each type was removed and cross-sectioned using standard metallographic techniques. Due to overlapping effects of scale growth, internal damage, spallation of corrosion products and salt along the edges of the samples, the metal loss or the corrosion depth measured based on the cross-sectional reduction is a more suitable way to assess the corrosion progress [20,21,22]. Six optical images throughout each sample were taken with a magnification of 50x (LEICA DMC 450). The remaining metal thickness was determined by averaging six perpendicular length measurements in each image using ImageJ software. The measurement began below the visible corrosion zone with internal damage in order to determine the remaining “good” metal.
Samples were characterized using scanning electron microscopy (SEM, Hitachi FlexSEM 1000 and Thermo Scientific Apreo 2 SEM), electron probe microanalysis (EPMA, JEOL JXA-8100) equipped with wavelength dispersive X-ray spectroscopy (WDS) and X-ray diffraction (XRD, Bruker D8 XRD Advance with Cu-Kα source). Back-scattered electron (BSE) images were taken using SEM to reveal the microstructures. One slurry-coated sample after hot corrosion for 300 h at 900 °C was analyzed using transmission electron microscopy (TEM). The TEM lamella lift-out was prepared by focus ion beam (FIB) equipped on an FEI Quanta 3D field emission gun (FEG) dual-beam SEM. TEM and scanning TEM (STEM) combined with energy-dispersive X-ray spectroscopy (EDS) were performed using a Tecnai F20 at 200 kV equipped with Oxford INCA EDS instrument. The measurements included EDS point measurements and element distribution maps. The identification and assumption of the phases present were performed based on the measured compositions within the WDS line scans and crystallography, correlating with existing phases in the PDF2 database as well as by calculations using the JMatPro-v10.2 software.
Results and Discussion
Microstructure of Coated Rene 80
A representative as-coated cross section and concentration profile of a pack cementation chromized Rene 80 is shown in Fig. 1. On the surface, a characteristic ~ 2 µm thick layer of Cr23C6 (confirmed by XRD, pdf2 file number used: 00–035-0783, not shown here) formed [13, 23, 24]. The high Cr activity from the deposition of an initial pure Cr layer on the material surface and a sufficiently high C activity from the C contained in Rene 80 enable the formation of the Cr23C6 layer, as shown before for other substrates [25]. Below the Cr23C6, the γ-Ni layer is enriched by up to 28 at.-% Cr which decreases until the nominal composition of Rene 80 is reached after ~ 15 µm, where the transition to the γ-γ’-microstructure occurs. Below the Cr23C6 layer inclusions of Al2O3 formed. This can be attributed to Kirkendall porosity by outward diffusion, which was then filled by Al2O3 during the heat treatment due to the Al content in Rene 80. An enrichment of Mn from the MnCl4 activator used up to 4.5 at.% within the diffusion layer is visible, but a significant incorporation of Cl could not be detected.
Characterization of the Cr/Si slurry-coated Rene 80 is described in detail in [18], and a representative as-coated cross section and concentration profile is shown in Fig. 2. The thickness of the diffusion layer and therefore the incorporated Cr reservoir is ~ 10 times larger for the Cr/Si slurry coating. The coating consists of a Cr- and Si-enriched γ-Ni matrix with precipitates of coarsened Si-rich M6C carbides and locally fine Ti-enriched MC carbides along the grain boundaries. The Cr content is at a maximum of 28 at.% in the outer zone of the diffusion layer and continuously decreases within ~ 150 µm, where the nominal composition of Rene 80 is approached.
Cr/Si-coated Rene 80 after [18]: a SEM cross section; b concentration depth profiles measured by WDS
In the pack cementation process, deposition occurs via gaseous Cr, while during the slurry process a Cr-containing liquid phase is created. The activity is expected to be significantly higher in the partially molten state, resulting in higher interdiffusion rates and a thicker diffusion layer. In addition, the Cr23C6 layer initially forms during the pack cementation process, which serves as a diffusion barrier, limiting the diffusion and thus the further enrichment of the surface with Cr [13]. With this novel slurry process, no Cr23C6 layer formed. Also, Harper et al. [26] reported the absence of a Cr23C6 layer during co-deposition of Cr and Si by the pack cementation process. Whether this is an effect of the addition of Si or the different application process cannot be conclusively assessed based on the results in the present work. However, it can be assumed that due to the initial concentration of Cr50Ni30Si20 in the slurry powder as well as the immediate melting, the C and Cr activities required for the formation of Cr23C6 on the surface are not sufficient.
Hot Corrosion Behavior
In Fig. 3a the measured metal loss from the analysis of the various cross sections due to hot corrosion is shown. The uncoated Rene 80 shows a huge average metal loss of 470 µm after 300 h, while the Cr pack cementation and Cr/Si slurry coatings show a loss below 80 µm. A reduction of > 0.5 mm by metal loss is an unacceptable reduction of load-bearing cross section in actual parts. Additionally, after 300 h, the Cr content below the corrosion zone was determined to indicate whether breakaway oxidation is imminent and to what extent the reservoir has been consumed. While Cr depletion is apparent for the uncoated and pack cementation-coated Rene 80 up to 80 µm, the subsurface region of the Cr/Si coating maintains a reservoir of ~ 20 at.% Cr (Fig. 3b).
Uncoated Rene 80
In Fig. 4 a cross section of uncoated Rene 80 with corresponding elemental distribution maps after 24 h of exposure is shown. Breakaway oxidation was accompanied by extensive internal oxidation and sulfidation. The present corrosion attack indicates rapid dissolution of the initially formed Cr2O3 scale by the molten Na2SO4, followed by continuous breakdown of the scale and S uptake into the alloy, which leads to extensive internal sulfidation. Because of the relatively low SO3 activities at 900 °C, basic dissolution of the oxides can be expected [5], where Cr2O3 scales would usually not be expected to demonstrate continuous self-sustaining hot corrosion attack [12]. However, Rene 80 is comparatively highly alloyed with refractory Mo and W for mechanical stability [27, 28]. Mo and W form very acidic oxides, and when these are dissolved in the molten salt, they strongly reduce the Na2O activity. This makes the salt locally very acidic, which can lead to the acidic dissolution and increase of solubility of Cr2O3 in Na2O [11, 29, 30]. The incorporation of W-oxides within the corrosion products is visible in Fig. 4, while Mo-oxides have probably evaporated [11]. It was reported that especially high Mo content, up to 4 wt.-% (such as Rene 80), can accelerate hot corrosion attack at 900 °C [31] because of the high acidity of this oxide.
After depletion of Cr, Ni is oxidized and reacts with the molten Na2SO4. It reprecipitates as the observed outer porous and unprotective NiO scale [11]. This had spalled off in Fig. 3, but was still attached in other areas of the sample. Below the corrosion front an inward-growing blister formed, which is a common shape of attack for hot corrosion type I and is already considered catastrophic corrosion [32]. At the interface, the alloying elements such as Ti and Al are selectively oxidized, causing a dissolution of the Ti- and Al-rich γ' Ni3(Ti,Al) (Fig. 3b).
Due to the lack of a protective oxide layer, S from the SO2-containing gas quickly diffused into the alloy and led to extensive internal sulfidation. Ni sulfides and Cr sulfides could be identified by EDS point measurements (not shown). Thermodynamically Ni sulfides are less stable when compared to Cr sulfides and Ti sulfides [33]. Based on exposures of Rene 80 at 1050 °C in air-2%SO2, Jalowicka et al. [34] concluded that first Ti sulfides form and after longer exposure times additional Cr sulfides form due to the reduced Ti activity by subsequent depletion and further transformation into Ti oxide. The additional Na2SO4 deposit accelerates the depletion of Ti and Cr, so that Ni sulfides were formed. As the corrosion progressed and the porous corrosion products on the surface spalled, all sulfides are oxidized and the released sulfur diffuses further into the alloy. Particularly, the existence of Ni sulfides (liquid under these conditions) accelerates the progression of the corrosion front [5].
Chromized Rene 80 by Pack Cementation
The pack cementation Cr-coated Rene 80 showed considerably slower and reduced corrosion attack when compared to the uncoated Rene 80, as indicated by the lower metal loss in Fig. 3. A cross section with corresponding elemental maps after 24 h is shown in Fig. 5. The initially formed Cr2O3 scale layer appears to have spalled off, which was evident by the spalled material in the crucible. Another indication that spalling occurred during the exposure is the pronounced surface porosity within the subsurface zone, which filled with resin during embedding of the samples and is visible as C-rich locations in the elemental map in Fig. 4b. It is known that Mn additions potentially increase the growth rates of Cr2O3 scales due to the simultaneous formation of MnCr2O4 spinel and, therefore, make the oxides more susceptible to spalling [35]. Since Mn is no longer visible in the EPMA maps in Fig. 5b after 24 h of exposure, Mn appears to have been enriched in the scale and promoted spalling. Due to the Cr reservoir, a self-healing of Cr2O3 was possible where Cr diffused outward and resulted in the observed Kirkendall porosity. After 24 h, a Cr reservoir remained, although it was significantly reduced and within 8 µm of the surface returned to a composition of ~ 18 at.-% Cr (measured by WDS). No remaining Cr-rich Cr23C6 phase is evident. Locally some S diffused inward and formed Cr sulfides, thus also contributing to Cr depletion. As Cr sulfide compounds are stable and trap S, further diffusion into the alloy is slowed down.
In Fig. 6 a cross section with the corresponding elemental maps after 300 h of exposure is shown. A dense Cr2O3 scale developed with a TiO2 scale at the metal/scale interface. TiO2 precipitates within the outer part of the Cr2O3 scale are also evident. Below the oxide scales is a growing zone of internal oxidation of Al (to Al2O3). Since the initially formed porosity disappears, it is likely that it filled with Al2O3. Underneath the oxidation zone, where the oxygen partial pressure is reduced, a zone of Cr sulfides and TiN (confirmed by EDS point measurements) formed. The Cr reservoir is completely used up after 300 h, and within the depletion zone of ~ 50 µm, the Cr content is reduced to below 8 at.-% within the first ~ 20 µm. No additional sulfides beyond Cr sulfides could be identified. However, with further depletion, the formation of detrimental nickel sulfides is expected. If salt deposits repeatedly occur and the Cr2O3 scale dissolves again, catastrophic breakaway oxidation would occur within a short period of time.
Cr/Si Slurry-Coated Rene 80
Figure 7 presents a BSE image of a Cr/Si-coated sample after 24 h of exposure. An external porous layer containing Ni, Cr, Ti, Al, O, S and Na (Fig. 7b) indicates that these are reaction products from the molten Na2SO4, which seems to have partly dissolved the initial Cr2O3 scale. Below this porous layer is a Cr2O3 scale with a continuous Al2O3 subscale. No large-scale corrosion attack and uptake of S into the material is evident. The partial dissolution of the oxide scale and subsequent outward diffusion of Cr to maintain scale growth led to a depletion of Cr within ~ 10 µm, where the Cr content of ~ 14 at.-% was measured (by WDS, approx. the base composition of the alloy). However, underneath the thin depletion zone a ~ 150 µm Cr reservoir is present, with a Cr content of at least 20 at.-%. Within the Cr depletion zone, a Si- and Ti–rich phase precipitated. According to thermodynamic calculations (JMatPro, not shown here) and from the given phase compositions in [36], this phase can be assumed to be cubic Ni16Si8Ti6.
Figure 8 shows a BSE image with a corresponding EDS line scan of the corrosion zone after 100 h. In the outer part of the oxide scale NiO precipitation within a NiCr2O4 matrix (confirmed by XRD pdf2 file number used: 00-075-0198 with the spinel structure) is evident. Progressing inward, the Cr content increases and a ~ 3 µm thick Cr2O3 scale can be measured (Fig. 8b). The structure of the oxide layers indicates that the partial dissolution of the oxide scale occurred by the basic fluxing mechanism. The oxides are dissolved by the molten Na2SO4 at the salt/oxide interface and reprecipitate according to their solubility gradient at a specific location within the melt. Since NiO shows a negative solubility gradient within the molten Na2SO4, it reprecipitates within the outer parts of the melt where the oxygen partial pressure is increased [12], as shown in Fig. 8a. In contrast to NiO, Cr2O3 shows a positive solubility gradient within the molten Na2SO4. Solubility of Cr2O3 increases with increasing oxygen partial pressure and therefore reprecipitation occurs at the oxide/salt interface [12]. This inhibiting behavior can be seen by the increasing Cr content within the oxide products in Fig. 7. For high Cr concentrations, like for the investigated Cr/Si slurry coating, the dissolution process slows down and is not self-sustained.
Where M6C precipitates are at the surface, these are oxidized and allow S uptake, as the formation of Ti sulfides can be seen at these locations. Mo- and W-rich carbides at the surface represent potential locations for increased corrosion due to their preferential oxidation and the local change of salt acidity by these oxides [11, 37]. However, the Cr/Si slurry coating effectively inhibits the attack and appears to hinder the spreading of the corrosion front.
After 300 h of exposure in Fig. 9, the microstructure is similar to the one after 100 h. After formation of the multilayer oxide scale, the corrosion process seems to follow steady state behavior. This is also supported by the recorded metal loss in Fig. 3. A TEM image of the interface between the oxide layer and metal is shown in Fig. 9 with the corresponding EDS maps. The presence of Ni in the oxide is limited to the outer region. Close to the interface between the Cr2O3 and the Al2O3 scale, an agglomeration of Si-rich precipitates was confirmed by STEM-EDS to be SiO2 which is most likely amorphous [38, 39]. Due to the decreased oxygen partial pressure from Cr2O3, Si is oxidized and forms SiO2 precipitates at the Cr2O3/metal interface according to Wagner’s getter mechanism [40]. With the coating containing ~ 5 at.% Si, it could be expected that the SiO2 particles would agglomerate over time to a dense subscale, as observed in [18, 38, 41]. Here, below the SiO2 particles Al2O3 formation is found. Under the current conditions, even the formation of a dense Al2O3 film becomes possible, most likely due to the “support” from SiO2 in lowering the oxygen partial pressure, although the discrete influence of the agglomerated SiO2 particles on the corrosion behavior cannot be conclusively assessed within the current study. However, one effect appears to be suppression of local, unprotective, internally oxidized Al2O3, as seen for the Cr2O3 scale formed after pack cementation. Afterward, in the Si-modified slurry coating, the Al2O3 subscale drastically slows down further potential outward diffusion of other elements.
TEM characterization of Cr/Si slurry-coated Rene 80 after 300 h at 900 °C. Left: Image with EDS maps. Right: Characterization of Cr sulfide. a STEM high angle annual dark field image. b STEM/EDS distribution of S, Cr and O. c Selected area diffraction (SAD) patterns of Cr sulfide acquired along < 211 > , < 310 > , and < 210 > zone axes compared with the simulated patterns. The lattice parameters used for simulation are a = 3.487 Å, b = 3.487 Å, c = 5.5278 Å, α = 90°, β = 90°, γ = 120°, space group P 63/m m c
In general, Al2O3 scales are known to be more effective diffusion barriers against O and S than Cr2O3 [33] and the subsequent oxidation and sulfidation of the Cr/Si slurry-coated Rene 80 was hindered by the continuous Al2O3 scale once it formed [5]. Sheybany and Douglass [42] reported rapid sulfidation for Cr2O3-forming alloys, while Al2O3-formers showed several orders of magnitude lower S uptake. Due to the local reduction in the oxygen partial pressure and increase in sulfur activity at the interface, the sulfides become more stable than the corresponding oxides [43]. The CrS film (confirmed by TEM in Fig. 8) instead of Cr2S3 and the precipitation directly underneath the Al2O3 subscale underline the prevailing low sulfur partial pressure. The Cr depletion below the oxidation zone also became less pronounced. As the presence of silicon is known to increase the diffusivity of Cr [44], most likely the Si additions had a beneficial effect in this reduction. As a result, also the previously precipitated Ni16Si8Ti6 phase dissolved. A Si depletion within the subsurface zone or diffusion of Si into the substrate could not be detected within the exposure time of 300 h, as the WDS measured Si content in the diffusion layer after exposure is comparable to the Si content in the as-coated state.
The Cr enrichment of the Cr/Si slurry enabled formation of stable CrS and reduced S-inward diffusion by the Al2O3 layer prevented internal sulfidation, in contrast to the single Cr2O3 layer of the pack cementation. The existing protective multiscale oxide layer and the remaining Cr reservoir after 300 h of exposure indicate that the protective effect of the coating would be retained even after longer exposure times or repeated Na2SO4 deposition.
Summary and Conclusions
The type I hot corrosion behavior of Rene 80 was investigated and compared to the results of chromized Rene 80 by the conventional pack cementation process and the recently developed Cr/Si slurry process. The uncoated Rene 80 showed rapid dissolution of the initial oxide scale, which led to breakaway oxidation with massive internal sulfidation throughout the sample within the first 24 h of exposure. Although the pack cementation Cr-coated Rene 80 showed extensive spalling of the oxide scale within the first 24 h, the Cr reservoir enabled healing of the Cr3O2 scale. With further exposure Cr depletion of the subsurface, internal oxidation of Al2O3 and a 30 µm zone of extensive internal sulfidation (stable Cr sulfides) could be observed. The pack cementation Cr coating significantly increased the hot corrosion resistance of Rene 80 and avoided catastrophic breakaway oxidation within 300 h. Nevertheless, with increasing duration and repeated salt deposition, catastrophic corrosion attack could be expected due to eventual Cr depletion.
The Cr/Si slurry-coated Rene 80 showed excellent type I hot corrosion resistance. Basic fluxing of the oxide scale could be inhibited by the protective reprecipitation of Cr2O3 along the oxide/salt interface. Due to the Si content present in the coating, agglomerated SiO2 particles formed underneath, which is believed to lead to a significant decrease of the inward diffusing oxygen, enabling the formation of a dense Al2O3 subscale. The resulting multilayer oxide scale represents an excellent diffusion barrier to O and S. Thus, instead of S uptake and internal sulfidation, the inwardly diffused sulfur was bound directly at the oxide/metal interface in the form of a stable CrS film.
Data Availability
No datasets were generated or analyzed during the current study.
References
F. S. Pettit, Oxidation of Metals 76, 1 (2011).
G. W. Goward, Surface and Coatings Technology 108, 73 (1998).
P. Kofstad and G. Åkesson, Oxidation of Metals 14, 301 (1980).
J. Stringer, Materials Science and Technology 3, 482 (1987).
H. J. Maier, T. Niendorf, and R. Bürgel, Handbuch Hochtemperatur-Werkstofftechnik, (Springer-Verlag, Wiesbaden, 2015).
R. Bianco, R. A. Rapp, and J. L. Smialek, JoES 140, 1191 (1993).
F. S. Pettit, G. H. Meier, M. Gell, et al., Superalloys 85, 651 (1984).
N. Birks, G. H. Meier, and F. S. Pettit, JoM 39, 28 (1987).
M. C. Galetz, in Superalloys, ed. M. Aliofkhazraei (InTechOpen, London, UK, 2015), p. 277.
P. Kofstad, Materials Science Forum 154, 99 (1994).
J. A. Goebel, F. S. Pettit, and G. W. Goward, Metallurgical Transactions 4, 261 (1973).
R. A. Rapp and N. Otsuka, ECST 16, 271 (2009).
R. Bianco and R. A. Rapp, Metallurgical and Ceramic Protective Coatings, (Springer, Netherlands, Dordrecht, 1996), p. 236.
J. R. Nicholls, JoM 52, 28 (2000).
B. Grégoire, G. Bonnet, and F. Pedraza, Surface and Coatings Technology 374, 521 (2019).
X. Montero, M. C. Galetz, and M. Schütze, JOM 67, 77 (2015).
M. Kerbstadt, M. C. Galetz, Verfahren zur diffusionsbeschichtung mit einem Cr-Si-haltigen Schlicker. Germany Patent 10 2022 112 093.7, 12 (Application Process pending) 05 (2022).
M. Kerbstadt, E. M. H. White, and M. C. Galetz, Materials 16, 7480 (2023).
Available online: www.hb-specialalloy.com/products/rene-80/ (Accessed 22 March 2024).
J. Sumner, A. Potter, N. J. Simms, et al., Materials at High Temperatures 32, 177 (2015).
T. König, X. Montero, and M. C. Galetz, Materials and Corrosion 70, 1371 (2019).
X. Montero, A. Ishida, T. M. Meißner, H. Murakami, et al., Corrosion Science 166, 108472 (2020).
D. Fähsing, C. Oskay, T. M. Meißner, et al., Surface and Coatings Technology 354, 46 (2018).
C. Oskay, T. M. Meißner, C. Dobler, et al., Coatings 10, 687 (2019).
D. Fähsing, Neuartige diffusions schichten zum oxidations schutz ferritisch-martensitischer Stähle in wasserdampfhaltigen Atmosphären. Shaker Verlag, PhD Thesis (2016).
M. A. Harper, R. A. Rapp, Chromized/siliconized pack cementation diffusion coatings for heat-resistant alloys, in Proceedings of the First International Conference on Heat Resistant Materials, eds. K. Natesan and D. J. Tillack, D.J. The American Society for Metals: Detroit (MI, USA, 1991), p. 379.
J. Safari and S. Nategh, Journal of Materials Processing Technology 176, 240 (2006).
S. Gao, J. S. Hou, K. X. Dong, et al., Acta Metallurgica Sinica 30, 261 (2017).
I. Gurrappa, Materials Science and Technology 19, 178 (2003).
R. A. Rapp, Corrosion Science 44, 209 (2002).
K. R. Peters, D. P. Whittle, and J. Stringer, Corrosion Science 16, 791 (1976).
N. Eliaz, G. Shemesh, and R. M. Latanision, Engineering Failure Analysis 9, 31 (2002).
D. J. Young, High Temperature Oxidation and Corrosion of Metals, (Elsevir, Amsterdam, 2008).
A. Jalowicka, et al., Materials and Corrosion 65, 178 (2014).
F. H. Stott, F. I. Wei, and C. A. Enahoro, Materials and Corrosion 40, 198 (1989).
M. Wambach, P. Ziolkowski, E. Müller, and A. Ludwig, ACS Combinatorial Science 21, 362 (2019).
F. Fatemi and F. S. Nogorani, Corrosion Science 196, 110031 (2022).
D. L. Douglass and J. S. Armijo, Oxidation of Metals 2, 207 (1970).
C. E. Lowell, Oxidation of Metals 2, 95 (1973).
C. Wagner, JoES 103, 627 (1956).
M. A. Harper and R. A. Rapp, Oxidation of Metals 42, 303 (1994).
S. Sheybany and D. L. Douglass, Oxidation of Metals 29, 307 (1988).
M. F. Stroosnijder and W. J. Quadakkers, High Temperature Technology 4, 41 (1986).
G. R. Johnston, High Pressures 14, 695 (1982).
Acknowledgements
This project has received funding from the European Union’s Horizon 2020 Research and Innovation Action (RIA) under grant agreement No. 958418 (COMPASsCO2). A Knowles acknowledges support from: UKRI Future Leaders Fellowship (MR/ T019174/1) and Royal Academy of Engineering Research Fellowship (RF\201819\18\158). The authors would like to thank G. Schmidt for EPMA analysis, C. Oskay for the support with pack cementation and the Centre for Electron Microscopy (University of Birmingham) for their support and assistance in this work.
Funding
Open Access funding enabled and organized by Projekt DEAL. European Union’s Horizon 2020 Research and Innovation Action (RIA), No. 958418 (COMPASsCO2), No. 958418 (COMPASsCO2), No. 958418 (COMPASsCO2), No. 958418 (COMPASsCO2), No. 958418 (COMPASsCO2), UKRI Future Leaders Fellowship, MR/ T019174/1,MR/ T019174/1, Royal Academy of Engineering Research Fellowship, (RF\201819\18\158), (RF\201819\18\158).
Author information
Authors and Affiliations
Contributions
M.K. contributed to conceptualization, methodology, investigation, and writing of the main manuscript text; K.M. contributed to TEM and SEM analysis, preparation of Figs. 8 and 9, and review and editing; E.M.H.W contributed to conceptualization, writing—review and editing, and supervision; A.J.K. performed review and editing and funding acquisition; M.C.G. contributed to conceptualization, writing—review and editing, supervision, project administration, and funding acquisition. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kerbstadt, M., Ma, K., White, E.M.H. et al. Novel Chromium–Silicon Slurry Coatings for Hot Corrosion Environments. High Temperature Corrosion of mater. (2024). https://doi.org/10.1007/s11085-024-10257-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11085-024-10257-8