Abstract
Reactive elements—REs—are decisive for the longevity of high-temperature alloys. This work joins several previous efforts to disentangle various RE effects in order to explain apparently contradicting experimental observations in alumina forming alloys. At 800–1000 °C, “messy” aluminum oxy-hydroxy-hydride transients initially formed due to oxidation by H2O which in turn undergo secondary oxidation by O2. The formation of the transient oxide becomes supported by dispersed RE oxide particles acting as water equivalents. At higher temperatures, electron conductivity in impurity states owing to oxygen vacancies in grain boundaries (GBs) becomes increasingly relevant. These channels are subsequently closed by REs pinning the said vacancies. The universality of the emerging understanding is supported by a comparative first-principles study by means of density functional theory addressing RE(III): Sc2O3, Y2O3, and La2O3, and RE(IV): TiO2, ZrO2, and HfO2, that upon reaction with water, co-decorate a generic GB model by hydroxide and RE ions. At 100% RE coverage, the GB model becomes relevant at both temperature regimes. Based on reaction enthalpy ΔHr considerations, “messy” aluminum oxy-hydroxy-hydride transients are accessed in both classes. Larger variations in ΔHr are found for RE(III)-decorated alumina GBs as compared to RE(IV). For RE(III), correlation with GB width is found, increasing with increased ionic radius. Similarly, upon varying RE(IV), minor changes in stability correlate with minor structural variations. GB decorations by Ce(III) and Ce(IV) further consolidate the emerging understanding. The findings are used to discuss experimental observations that include impact of co-doping by RE(III) and RE(IV).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Durability of functional alloys that serve at high temperatures relies on the formation of a protective slow-growing oxide scale to avoid breakaway oxidation. Aluminum and chromium constitute the main scale-forming elements in the commonly employed iron-, cobalt-, or nickel-base alloys. Greater thermodynamic stabilities of Al2O3 and Cr2O3 as compared to the oxides of the more noble base metal render both to form and enrich at the alloy surface, thereby providing the base metal protection from the harsh environment. When both Al and Cr are present in the alloy, Cr acts as 3rd element in that under oxidizing conditions, transient Cr2O3 inhibits internal oxidation of aluminum and facilitates the formation of the more stable alumina [1,2,3]. The 3rd element effect is most effective at intermediate temperatures, i.e., 800–1000 °C. Indeed, due to kinetic considerations as well as absence of competitive structures to corundum, the formation of chromia is understood to act template for α-Al2O3. At very high temperatures, i.e., higher than 1200 °C, α-Al2O3 becomes increasingly more readily accessed.
The electrochemical processes involved in scale growth include among others electron conduction. Here, the alloy/oxide interface is acting anode from which electrons are supplied to the outer oxide surface where the cathodic O2 reduction reaction takes place [4]. Outward diffusion of metal cations and inward diffusion of oxygen anions ensure the positive charge of the anodic interface and corresponding negative charge at the outer cathodic oxide surface to be kept at steady-state. The outward diffusion of cations is unwanted as it renders the receding alloy/oxide interface prone to void formation, which in turn compromises scale adherence. This is one of the effects that are countered by small additions (less than 1 wt%) of so-called reactive elements REs to the alloy, e.g., Y, Zr, Hf, and Ce. The enigmatic roles that these additives, sometimes termed magic dust [3, 5, 6], have been the topic of studies ever since the beneficial effects were first reported some 80 years ago; see [7]. Some 30 years ago, it was concluded that among these effects, mitigation of alumina scale spalling by improving scale/alloy adhesion was separable from the rest [8, 9].
The quest for understanding of the RE effect in alumina formers, first championed by Stringer, was consolidated and extended in studies by Pint, Heuer, and others. Four beneficial roles of reactive element additions have been formulated:
- 1.
Elemental RE dopants are crucial inside the alloy where they offer sinks, e.g., the sulfur, carbon, nitrogen, and oxygen, that have remained in the matrix from the manufacturing stages. Under cyclic thermal conditions, sulfur, in particular, is known to enrich at the oxide/alloy interface where it impairs scale adhesion [3, 8,9,10,11,12,13].
- 2.
At the alloy/oxide interface, elemental RE dopants become oxidized ending up decorating the oxide grain boundaries. This way, inhibition of grains coarsening is achieved, which in turn supports parabolic scale growth, i.e., suppresses the subparabolic kinetics owing to loss of grain boundary density [14, 15]. This is beneficial, in particular, at lower temperatures as it accelerates protective alumina scale growth [16,17,18,19,20,21,22,23,24].
- 3.
RE doping achieves suppression of outward diffusion of Al3+ ions, thereby producing a well-adherent oxide scale. To explain this effect, a dynamic-segregation mechanism has been proposed, replacing outward diffusion of Al3+ by that of RE ions as driven by the oxygen activity gradient across the scale [6, 11, 23, 25,26,27,28,29,30].
- 4.
The impact of RE doping was revisited by Heuer et al. [31, 32]. Thermal growth of alumina scale was subject to a fully electrochemical contextual analysis, including electronic conductivity properties as well as oxygen-activity-dependent ionic transport with bearing on the RE effect. In particular, Y2O3-attenuated electron conductivity was inferred based on inspirational studies of Σ7 grain boundaries by means of first-principles density functional theory [32]. In a subsequent study, the Σ7 grain boundary was subject to extended in-depth analysis by means of modeling [33]. Building on the latter study, but notwithstanding that high temperatures increasingly disfavor cooperative processes, their emerging working hypothesis regarding the RE effect still envisioned the moderation of cooperative migration at high temperatures, mainly as jogs along grain boundary disconnections, but this time also together with other conceivable cooperative mechanisms, the possible viability of any or all to be assessed by atomistic simulations [34].
And still, elemental additions of RE in the alloy must be kept low owing to unwanted excessive internal oxidation of RE inside the alloy or due to pegging [35]. In both cases, the oxygen activity originates from the dissociation pressure of alumina at the alloy/oxide interface.
Besides RE additions in the alloy in elemental form, RE oxide dispersions are common additives in oxide-dispersion-strengthened ODS alloys. At first, somewhat surprisingly, the impact of the RE oxide additive on scale growth bears great similarity to that of the elemental RE additives. However, in as much as elemental RE (e.g., Y, La, Zr, Hf) are very strong reducing agents, it readily follows that the oxidizing environment will render these elemental additives oxidized. To this end, the present study is part of a comprehensive effort that focuses on the impact on oxide scaling that any RE oxide particle residing at the outer surface of the component may have. Thereby, a complementary origin for the observed transient RE decoration of alumina grain boundaries is arrived at.
As longstanding as the roles of RE in oxidation of alumina formers, is the impact of water in high-temperature oxidation. Crucial effects include the incorporation of hydrogen in ionic form in the oxide, rendering it defect rich, which in turn controls the transport properties during scale growth [36]. Moreover, it was stated in [37] that even very dry gas mixtures—achieved, e.g., by employing phosphorous (V) oxide as drying agent—contain ~ 3 ppm H2O(g) which suffices to dominate or greatly influence the resulting defect structure in Y2O3 upon oxidation in nominally dry oxygen.
In general, conclusions as to what controls the formation of protective alumina, including RE effects, are based on scaling studies at high temperature. This is because as faster the oxidation is, the higher is the temperature, as scaling rates are often dominated by the activation energies for diffusions according to the Arrhenius equation. Thus, it was gratifying to unravel a crucial interplay of water and reactive elements at intermediate temperatures by combining experiment and modeling [38]. Oxide scale formation by water under nominally reducing and “dry” conditions was achieved in carefully controlled exposure experiments in 95%N2, 5% H2, 35 ppm H2O, and at T = 1173 K and 1273 K. The nature of said oxide scale was subject to careful analysis. Rapid oxide scale formation on ODS FeCrAlY was found to involve two stages: (1) initial oxidation where water vapor comprises the only oxidant in conjunction with Y2O3 particles jointly supporting a rapidly growing transient nanogranular “messy” aluminum oxy-hydroxy-hydride film and (2) a second stage where the transient oxide in turn becomes oxidized. Availability of yttria was found to control the local thickness of the transient “messy” oxide and contact with the notion of local overdoping/overdosing was also made. Preference for monodisperse submicron-sized yttria particles was advocated to avoid build up of stress and subsequent scale cracking owing to large local variations in scale thickness.
Driven by the oxidation of aluminum, in the first stage, the yttria additive facilitates water to, in effect, permeate the oxide scale. Hydrolysis followed by proton-assisted inward diffusion of oxygen ions pins an inner cathode to the vicinity of the alloy/oxide interface where proton reduction takes place, being complementary to the alloy oxidation. Crucially, hydrogen is disposed as hydride ions in oxygen vacancies H−@V**Oat an “inner cathode” in the vicinity of the mobile alloy/oxide interface. Thus, the transient defect-rich alumina nanograins formed become terminated jointly by Y3+, OH− and said H−@V**O.
Subsequently, in the second stage, the resulting transient oxide film is understood to oxidize to finally produce a well adherent protective α-Al2O3 scale. Yttrium enrichment is observed—in that yttrium aluminum garnet YAG nanograins form and grow in the upper oxide—simultaneously with the α-Al2O3 formation and grains coarsening in vicinity of the alloy/oxide interface; see Scheme 1. This outward diffusion of RE ions replacing that of Al3+ is reminiscent of the dynamic-segregation mechanism proposed by Pint [29] for high temperatures.
Reactive element-assisted scale formation at intermediate temperatures disentangled: 1. Fast formation of a nanogranular messy oxide owing to aluminum oxidation by water. RE oxide assisting in scale permeation of water, in the form of H+ and OH−, results in RE ions co-decoration of alumina grain boundary interfaces. Residual hydrogen, being accumulated as hydride ions in charged oxygen vacancies, renders the messy oxide partially reduced. 2. The partially reduced messy oxide undergoes oxidation by O2, rendering the resulting RE-decorated aluminum oxy-hydroxide unstable. 3. RE dependent rate of reprecipitation controls the rate of grains coarsening. The dynamic blocking effect combines 2 and 3 as the cathode process is taken to drive the outward diffusion of RE cations
Indeed, in as much as the oxide grain boundaries constitute rapid diffusion paths, the time evolution of grain boundary density change becomes decisive for the rate of scale growth [14, 15]. A crucial role of the RE becomes to disallow early grains coarsening—by the Y3+ decoration of nanograins—the consequence of which is the observed initial high rate of scale growth. The inwards growing initial “messy” oxide—besides being virtually free of voids—becomes sufficiently thick to ensure its transformation into the requested slow-growing protective α-Al2O3 scale by the secondary oxidation step, and this despite low Al content in the alloy (5 wt%).
This mechanism in two stages—rapid initial incomplete oxidation by H2O(g) + Y2O3 followed by an oxidation step to achieve a well adherent oxide scale—was validated by combining experiments and first-principles modeling. Building on this mechanistic understanding and modeling procedure, the purpose of the present study is twofold, in that (1) it explores the degree of universality of the concerted mechanism by yttria being replaced by other RE oxides, and (2) generic insights are sought for how reactive elements may regulate the outward diffusion of cations by RE ionic charges and radii, thereby causing time-dependent structural modifications in the grain boundaries.
Inspirational in our effort to unravel the crucial cooperation between water and RE at the early stages of oxidation of alumina formers is a similar phenomenology for ODS FeCrAlY [38] and ODS CoCrAlY [39]. This search for generic mechanistic insight from first principles benefits from maximum control of the model at hand. That, in turn, allows for systematic considerations in an ideal manner, here, both with regard to RE and composition of the model interface between alumina grains. Yet, the relevance of the emerging understanding—to real applications—must be established by means of experiments.
The present study is based on the properties of electroneutral monolayers of RE ions and hydroxides co-decorating interfaces between α-Al2O3 slabs, cf. [38]. It provides energy landscapes as well as structural descriptors that emerge from systematically varying the monolayer compositions. The reactive elements considered comprise Al3+, Sc3+, Y3+, La3+ and Ti4+, Zr4+, Hf4+. These are classified according to oxidation numbers. Also, relevance of Ce3+ and Ce4+ in this context is explored. Contact between scale growth at intermediate and high temperatures is inferred in case of completely dehydrated grains boundaries, i.e., corresponding to 100% RE decorations.
Modeling Considerations
The density functional theory DFT based modeling approach described in [38] is reiterated here. Thus, steady-state yttria-assisted permeation of the alumina scale by water to achieve overall oxidation of aluminum at the alloy/oxide interface was validated employing a hydroxylated alumina model interface between 1.5-nm-thick lamella with a variable degree of co-decoration with Y3+ as mediating transients. Here, that understanding is generalized as two sets of RE are considered, having oxidation states III and IV, i.e., RE(III): Al3+, Sc3+, Y3+, La3+, Ce3+ and RE(IV): Ti4+, Zr4+, Hf4+, and Ce4+.
Caution is commonly warranted when employing present day’s implementations of DFT to systems that may possess accessible local and delocalized Kohn–Sham states. This is owing to the self-interaction error SIE in DFT that favors the latter over the former. This commonly renders band gaps underestimated as valence bands are generally more localized than the conduction bands. Here, this is emphasized for Ce(III) owing to the small size of the occupied 4f orbital. Consequently, any semiconductor properties associated with Ce(III) are disfavored as compared to the erroneous semi-metal. However, the electron density of said semimetallic states approximate the nominal VB states well, and thus the external potential, as seen by comparing computed crystal structure to the experimental for Ce2O3. Having said this, still the total energy of the faulty semimetallic 4f band suffers from the SIE. In as much as the occupation of the 4f states does not change during the chemical reactions considered, the impact of the SIE on the reaction energies approximately cancels. In the present study, this condition is satisfied throughout. Hence, while the description of Ce(III) comes with greater uncertainty than any of the other RE(III) and RE(IV) ions considered, it is deemed sufficient in the present classification study.
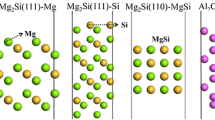
In the present study Al(s), RE(III) or RE(IV) oxides and a fully hydroxylated interface (see Fig. 1) between nanogranular alumina were taken as reactants. These are described by a generic periodic slab model that employs hydroxylated interfaces between 1.5 nm thick α-Al2O3 lamella. The following reactions were considered for RE(III) and RE(IV), respectively:
In R1 and R2, “H2O”@GB reflects the fact that the hydroxylated interface is owing to hydrolysis. In R2, \(V_{\text{Al}} @{\text{GB}}\) refers to the Al vacancy which is generated for every triad of \({\text{RE(IV)}}\) that is introduced. The obtained reactions energies were offset by the hydrolysis reactions that maintain the hydroxylated interfaces [16]. As to the fate of hydrogen, a lower bound to the exothermicity is assumed by employing formation of H2(g) where in fact H−@VO**, hydrogen pick-up in the alloy, or hydrogen being oxidized to form water are the relevant hydrogen sinks. The reaction energies were equated with reaction enthalpies throughout, as reactants and products vibration energies as well as corresponding entropy were taken to cancel. Lower bounds to the Gibbs free energy landscape for the co-decoration of grain boundaries by Y3+, and hydroxides were estimated by taking the loss of translational entropy of water, 144 JK−1 mol−1 (~ 1.5 meVK−1) to comprise the only entropy change of the reaction. Said lower bound is because the increase in configurational entropy of the resulting mixed solid, i.e., RE-decorated nanogranular aluminum oxy-hydroxy-hydride “messy” scale is neglected. Thus, an estimated upper bound for the temperature where the co-decoration of Y3+ and hydroxides is spontaneous, i.e., ΔG ≤ 0, may be formulated according to
Robustness of the understanding arrived at employing said hydroxylated interfaces between 1.5 nm thick alumina lamella co-decorated with Y3+, which was validated by removing the alumina scaffold all together employing a quasi-2D Al(III)OOH structure, based on Gibbsite, to model the hydroxylated interface.
The spin-polarized DFT calculations in this study were performed using the CASTEP code within the Materials studio 6.0 suite [40]. The PBE-GGA functional was employed for all calculations. Non-local Vanderbilt ultrasoft pseudopotentials have been used to describe core electrons together with a 550 eV energy cutoff, and the Brillouin zone was sampled with 0.04 Å−1 k-points separation employing a 3 × 3 × 2 sampling mesh [41]. The convergence criteria included 10−3 Å (displacement) and 10−5 eV/atom (energy). Impact of London interaction [42] on the structure and energetics of co-decoration was previously found to be negligible for Y3+. Here, this was found to hold true also for Zr4+.
Results
Two sets of reactive elements RE(III) and RE(IV) were investigated, in order to assess the degree to which the emerging understanding regarding the yttria effect at intermediate temperatures, as reported in [38], is indeed universal. Conversion of RE oxide particles to RE ions decorating alumina grain boundaries driven by aluminum oxidation by water according to R1 and R2 is summarized in Fig. 2a, and is converted into corresponding Gibbs energy topographies. All reaction enthalpies in Fig. 2a come out exothermic, originating from the high enthalpic drive of aluminum oxidation by water. The stability separation between RE(III) and RE(IV) bundles in Fig. 2a originates from the fact that \(\frac{3}{2}{\text{H}}_{2}\) evolves from \(\frac{3}{2}{\text{H}}_{2} {\text{O}}\), while each RE(IV) is associated with the evolution of \(2{\text{H}}_{2}\) from 2 \({\text{H}}_{2} {\text{O}}\). What truly differs between the +III and +IV bundles is the larger variation in reaction energies among the former set. This spread is consistent with correspondingly larger variations in grain boundary width as quantified by the O–O distances across interfaces between alumina slabs; see Figs. 3 and 4 for +III and +IV, respectively. It is observed that with the exception of Ce(IV), regardless of RE(IV) size, the grain boundary width remains virtually unchanged. Indeed, complexity in variations of GB widths as function of RE(III) concentration is intuitive, i.e., owing to varying degrees of compatibility of cation sizes with the coexisting structure former, comprising the hydroxides offering intergranular hydrogen bonding. The absence of such complexity in case of RE(IV) may at first be counterintuitive. It may partly be understood to originate from the condition of charge neutrality in the alumina scaffold enforcing every three RE(IV) residing in the interface to coexist with a nominal cationic vacancy. Thus when cation radius changes R(Ti4+) < R(Zr4+) ≈ R(Hf4+) < R(Ce4+) the corresponding buildup of stress may be relaxed by lateral deformations in case of the three former and thus no increase in O–O distances across the grain boundary is needed. An exception to this rule is offered by Ce4+, the ionic radius of which is significantly larger than that of the other RE(IV) ions considered in that R(Ce4+) ≈ R(Y3+). Thus, it comes out intermediate between the two classes. Indeed, the inability to achieve lateral relaxation in case of RE(III) is reflected in the observed larger variations in GB widths related to RE(III) ion size dependence. The mean O–O distances increase with increasing cation size (R(Al3+) < R(Sc3+) < R(Y3+) < R(La3+) ≈ R(Ce3+) becomes especially clear when comparing Y3+ and La3+. While the smaller RE(III) cations may be accomodated, the increasing structural mismatch of the larger RE(III) cations drives the separation between interface planes, consequently causing stresses owing to the GB decoration.
a Reaction energies and b temperatures for the RE/H2O co-decoration reaction, cf. R1 and R2. The temperature topographies determine the maximum temperatures for spontaneous formation for formation of RE-decorated grain boundaries from the corresponding RE oxides, see Fig. 1b. Overall, with the exception of Ce4+ (see more below), the +III (full lines) and +IV cations (dashed lines) are separated in bundles with the +III cations showing the larger variations in stability, especially at lower coverages
Two properties related to the impact of RE(III) decoration on the effective grain boundary width measured by the mean oxygen–oxygen distance across the two interface planes. a Mean GB widths and b corresponding standard deviations of the local oxygen–oxygen distances are displayed as function of RE(III) coverage. Larger cations cause larger GB widths
Impact of RE(IV) decoration on the effective grain boundary width measured by the mean O–O distance across the two interface planes. a Mean GB widths and b corresponding standard deviations of the local O–O distances are displayed as function or RE(IV) coverage. Note the minor variations in both graphs, the exception being Ce4+ and this owing to its significantly larger ionic radius
Besides interatomic distances across grain boundaries, the difference between RE(III) and RE(IV) decorations are manifested in connectivities among potentially viable interstitial sites for cation accommodation as well as mobility. Here, this is visualized for 100% coverage for a selected electrostatic potential isosurface EPI, see Fig. 5. For the RE(III) ions, Sc3+, Y3+, La3+, little effect of ionic radius increase on the EPI is observed. The similarly disconnected EPI among RE(III) is consistent with structural changes owing to an increase in radius to be manifested as changes in the grain boundary widths. However, for the RE(IV) ions, i.e., Ti4+, Zr4+, Hf4+, the EPI goes from being interconnected in case of Ti4+ to increasingly fragmented in case of Zr4+ and Hf4+. It offers a further manifestation of lateral relaxations in the interface absorbing the impact of RE(IV) cation size increase.
Negative electrostatic potential isosurfaces EPI:s (gray) for the RE-decorated interfaces. Front view (left). Side view (right). Localized EPI:s for RE(III) Sc3+ < Y3+ ~ La3+, are contrasted by more extended EPI:s among RE(IV) Ti4+ > Zr4+ ~ Hf4+. (Red balls: Oxygen; green balls: RE; Purple balls: Aluminum) (Color figure online)
Discussion
Strategies to achieve control of oxide scaling on alumina formers at high temperatures > 1200 °C by employing elemental RE dopants are described in the Introduction section. In addition, the interplay between water and RE oxide particles that reside on the outer surface of the specimen has been shown to impact the scaling in a major way at intermediate temperatures, i.e., 800–1000 °C [38], where electrochemistry is expected to be slow. The observed promotion is owing to yttria assisting in the oxidation process whereby the alloy becomes oxidized by water. At early stages, the observed yttria decoration results from the yttria acting “water equivalent” supporting the oxidation process, where the Y3+ ions end up decorating the resulting aluminum oxy-hydroxy-hydride nanograins of a “messy oxide” that grows exclusively inwards owing to the inner cathode; see Scheme 1. This generic process exhibits variations in stabilities mainly among RE(III) ions and at low RE coverages; see Fig. 2b. At subsequent stages, the Wagnerian oxidation channel owing to O2 reduction becomes increasingly more important, whereby the resulting RE-decorated aluminum oxy-hydroxy hydride nanograins become oxidized by O2 acting outer cathode. This secondary oxidation of the “messy” oxide renders nanograins to fuse and cause the RE cations in the grain boundaries to diffuse back into the outer oxide toward the outer cathode, reminiscent of the dynamic-segregation mechanism of Pint [29]. The fact that smaller RE cations do not block outwards diffusion of aluminum to the same extent as the larger cations have been attributed to the former partially dissolving in the alumina matrix, disallowed owing to ionic radius mismatch for the larger cations forcing these to reside in the grain boundaries. Indeed, during alumina grains coarsening in the inner part, precipitation of RE-rich oxide particles was observed in the outer part of the oxide scale [38]. As a consequence of the continuous loss of grain boundary density, the initial parabolic scale growth kinetics goes subparabolic. Arguably, the stability of the transient RE-decorated grain boundaries suppresses this transformation, thus rendering the rate of oxide growth greater for RE(IV) decoration than for RE(III) and greater for RE(III) decoration than in absence of RE decoration, cf. Figure 2. The overall change in scale thickness at intermediate temperatures ~ 800–1000 °C was modeled in [15, 38], cf. Figure 6a,
i.e., early parabolic \(X \propto \sqrt t ,\) and late \(X \propto \sqrt {\ln (t)}\). Note that in general the different dynamic-segregation effects of different RE additives affect the grain boundary diffusivity differently, thus causing the rates of mass gain to differ also in the parabolic regime, i.e., prior to grains coarsening.
Mass gain curves. a Modeling behavior at intermediate temperatures, cf. Equation 1. Three cases are illustrated: undoped (bottom), impact of Y2O3 OD (middle), and HfO2 OD (top) in FeCrAl alloy. Undoped FeCrAl shows lowest mass gain owing to grains coarsening initiated very early in the absence of RE decoration of grain boundaries. It is followed by Y3+ (or La3+) and Hf4+ (or Zr4+) decorated grain boundaries FeCrAl, i.e., in stability order (cf. Figures 2, 3, 4). b Modeling isothermal behavior at high temperatures, cf. Eq. 2, experiment is [23]. Linear is \(\Delta m \propto \sqrt t .\) Sublinear is \(\Delta m \propto t^{{\frac{1}{3}}}\). Undoped FeCrAl shows parabolic scale growth, while RE decoration inhibits transport in grain boundaries. Insets illustrate the opposite impacts of RE on scale growth at a intermediate and b high temperatures
This scaling provides the initial conditions for scale growth at higher temperatures in that said transformation reflects the further increased relevance of the outer cathode, i.e., by O2 reduction. It is achieved by the enhanced electronic conductivity across the scale emerging from the top-of-valence-band to the bottom-of-conduction-band electronic excitations that in turn become facilitated by chemical disruptions in high-angle grain boundaries [32,33,34], and/or by employing oxygen vacancies as generic impurity states in the band gaps of the grain boundaries [2, 16, 38]. In [2], bipolaron-mediated redox processes among VO sites were shown to drastically enhance VO mobility and thus to facilitate thermal oxide growth. The change in scaling conditions is captured by the superparabolic–cubic model [15]; see Fig. 6b,
Interestingly, Eq. 2 requires initial grain boundary density increase to reach a maximum at \(t = \frac{5}{4}t_{0}^{c}\), prior to subsequent grains coarsening to occur. Indeed, the two asymptotic limits for Eq. 2 are the superparabolic \(X \propto t^{{\frac{2}{3}}}\) for \(t \ll t_{0}^{c}\) and the cubic \(X \propto t^{{\frac{1}{3}}}\) for \(t \gg t_{0}^{c}\), the latter independent of \(t_{0}^{c}\).
On activation of the outer cathode at high temperatures, the dynamic-segregation mechanism [29] too is activated. The former is because electron hopping becomes increasingly accessible, while the latter infers that besides the inward diffusion of anions, the complementary outward diffusion of aluminum ions becomes replaced by that of RE ions [25, 28, 29]. The outward migration of RE is indeed reported again and again in the literature; see, e.g., [35]. A feedback effect of RE ions attenuating the electron conduction in turn is arrived at. Indeed, in the absence of RE, early parabolic scaling is observed, while cubic oxide growth is found upon RE doping. These observations support RE-induced oxidation slowdown by pinning impurity states in the band gap, cf. [2] and also [32,33,34]. Greater suppression by RE(IV) than RE(III) is consistent with greater stability in case of the former, cf. Figure 2. It is concluded that at intermediate temperatures, REs assist the oxidation when water acts oxidant. At elevated temperatures though, REs suppress the oxidation by O2 (i) by reducing electron conductivity and (ii) by suppressing the outward diffusion of aluminum ions. The latter renders the oxide scale inward growing owing to inward diffusion of oxygen ions, that in turn results in apparent grains coarsening as reflected in the cubic mass gain curves [23]; see Fig. 6b again. Subsequent reprecipitation of RE oxide particles promotes the further coarsening of alumina grains.
It is inferred from Figs. 2, 3, and 4 that reprecipitation of Ce4+ or RE(III) in the outer oxide is faster than for Zr4+ or Hf4+. This is owing to the buildup of additional stress in case of the former as manifested in the RE concentration dependence of the grain boundary width (see Figs. 3, 4). Indeed, a potentially crucial consequence is that the decay of grain boundary density becomes slower for Zr4+ and Hf4+ than for Ce4+ or RE(III). Thus, the water permeation channel is kept open longer than in case of both RE(III) and Ce4+ allowing for the “messy oxide” to grow thicker. Repeatedly, the mainly non-electrochemical channel sustains a well-adherent scale by maintaining an inner cathode in vicinity of the alloy/oxide interface. This renders obsolete the electrochemical requirement of compensating outwards diffusion of cations in order to maintain constant the steady-state charge separation across the oxide scale. It is noted here that would the RE source be elemental and reside in the alloy matrix, “pegs” would readily form owing to internal oxidation. In contrast, oxidation due to the interplay of water and RE oxide is terminated from below by the RE oxide grain size.
Said absence of grain boundary width driven formation of RE-rich particles in case of Zr4+ and Hf4+, while present in case of RE(III), as well as for Ce4+, offers the opportunity to optimize the scale adhesion property while maintaining a slow-growing oxide. Thus, it is tempting to propose improved protective properties to result from co-doping alumina formers by RE(III) and RE(IV) oxide dispersions also in line with [23, 43]. These authors found that all REs decorate grain boundaries independent of doped separately or being co-doped. Yet, oxide scale growth kinetics showed superior behavior by co-doping with RE(III) and RE(IV). First and foremost, it is the time evolution of the grain boundary density that is modified by tuning this mixture. In as much as scale growth benefits from suppression of alumina grains coarsening, this is achieved by RE(IV) decoration of grain boundaries. Interestingly, in locally mixed co-decorations of grain boundaries by RE(III) and RE(IV), the former may benefit from the nominal Al vacancies, see R2, generated by the RE(IV) ions, i.e., RE(III) decoration-induced stress owing to grain boundary width expansion is avoided. Thus, owing to zirconium or hafnium dopants, e.g., YAG formation and corresponding alumina grains coarsening is suppressed in favor of sustained grain boundary co-decoration of Y3+ and Zr4+ or Hf4+ (Fig. 2b). Also based on stability arguments, mixing different REs may indeed be beneficial. In as much as La(III)@GB or Ce(III)@GB forms more readily from their corresponding sesquioxides than does Y@GB, the former may assist the latter at low RE coverage, cf. Figure 2. While both Ce2O3–Ce(III)@GB and CeO2–Ce(IV)@GB couples have been addressed in the present study, it is reasonable to assume, based on thermodynamics as displayed in, e.g., the Ellingham diagram, that Ce2O3–Ce(III)@GB is the relevant one at early stages in the oxidation process. This is because—at a given pO2—oxidation of Ce(s) to form Ce2O3(s) precedes oxidation of Al(s) to form Al2O3(s) that in turn precedes the oxidation of Ce2O3(s) to CeO2(s). Finally, also based on thermodynamic considerations it is tempting to assume Ce(III)@GB to become oxidized by “H2O”@GB [44, 45]. However, repeating the previous reasoning renders Al as the actual reducing agent and consequently the Ce2O3–Ce(III)@GB couple prevails in grain boundaries that maintain “water permeation” to the alloy/oxide interface in case of cerium as reactive element.
Summary and Conclusion
Possible complementary reactive element effects in alumina formers at high and intermediate temperatures were described.
At high temperatures, alumina scale formation was explained by conventional Wagner theory, i.e., outer cathode: reduction of O2 at the gas/oxide interface, anode: oxidation of metal at the alloy/oxide interface, albeit electron and ions transport across the scale are confined to oxide grain boundaries. The RE additives offer control by suppressing the GB-mediated transport.
At intermediate temperatures, Wagner theory supports only very slow oxide growth by the outer cathode. Observed rapid oxidation is owing to RE oxide effectively facilitating water to permeate the scale to reach an inner cathode. RE oxide additives thus support scaling in that it aids the oxidation by water to build a sufficiently thick and well-adherent transient oxide scale.
The two properties become coupled in thermal cycling applications, e.g., jet turbines, where the conditions for transient oxide formation reappear in each cycle and therefore repeatedly set the stage for the high-temperature oxidation. An emerging class of applications concerns alumina formers as nuclear fuel cladding materials to achieve improve resilience also under accident conditions by utilizing the slower oxidation kinetics of FeCrAlRE alloys, i.e., by the formation of chromia under working conditions and increasingly alumina as temperature rises—as compared to conventional zirconium alloys [46]. Crucially, by safeguarding the RE effect at intermediate temperatures, scale spalling at high temperature is avoided.
Universality as to the interplay between reactive elements and water, see Scheme 1 and [38], was validated, in particular, for intermediate temperatures.
- 1.
RE(III) causes significant increase in GB width with increased ionic radius, suggesting oxidation-driven buildup of stress to support rapid RE oxide reprecipitation and corresponding grains coarsening. Thus, rapid transition from parabolic to subparabolic scale growth kinetics is inferred.
- 2.
RE(IV), with the exception of Ce4+, very small changes in GB widths are observed upon increasing RE ions radii. Owing to the greater stabilities, slower RE oxide reprecipitation is expected allowing more enduring parabolic scale growth kinetics
- 3.
Thermodynamic considerations under steady-state conditions imply the Ce speciation to comprise the Ce2O3–Ce(III)@GB couple—the former at the outer oxide interface and the latter in the grain boundaries—for as long as these effectively permeate water to the alloy/oxide interface where aluminum is oxidized.
- 4.
It is inferred that mixing different RE oxide dispersions may provide enhanced control of initial “messy oxide” formation at intermediate temperatures. Indeed, in as much as Y(III)@GB from Y2O3 comes out non-spontaneous above 500 °C at low coverage, this may indeed be compensated by both the La2O3–La(III)@GB and the Ce2O3–Ce(III)@GB couples.
- 5.
At later stages, the duration of parabolic scale growth preceding grains coarsening may be achieved by tuning the resilience of the grain boundary density by co-decoration of RE(III) and RE(IV), i.e., whereby the drive for secondary RE oxide reprecipitation becomes suppressed as RE(III) may utilize the vacancies generated by the RE(IV) to some extent alleviate the inherent stress associated with RE(III)@GB.
- 6.
RE(IV) decoration of alumina grain boundaries enforces aluminum vacancies owing to the charge neutrality constraint enhancing RE(IV) mobility while at the same time suppressing Al3+ mobility whereby a dynamic-segregation effect is achieved.
Thus, alloys with improved corrosion resistance are anticipated from the fact that superior control of oxide scale growth kinetics may be achieved along two different routes: by the interplay between water and the RE oxide dispersion at intermediate temperatures and by elemental RE dopants in the alloy at high temperatures.
References
Z. G. Zhang, F. Gesmundo, P. Y. Hou and Y. Niu, Corrosion Science48, 741 (2006).
V. Babic, C. Geers and I. Panas, RSC Advances8, 41255 (2018).
D. P. Whittle and J. Stringer, Physical and Engineering Sciences295, 309 (1980).
C. Wagner, Corrosion Science5, 751 (1965).
F. A. Golightly, F. H. Stott and G. C. Wood, Oxidation of Metals10, 163 (1976).
F. A. Golightly, G. C. Wood and F. H. Stott, Oxidation of Metals4, 217 (1980).
J. Stringer, Materials Science and Engineering: A120–121, 129 (1989).
D. Naumenko, B. A. Pint and W. J. Quadakkers, Oxidation of Metals86, 1 (2016).
V. Kochubey, D. Naumenko, E. Wessel, et al., Materials Letters60, 1654 (2006).
G. J. Tatlock, H. Al-Badairy, R. Bachorczyk-Nagy and R. Fordham, Materials and Corrosion56, 867 (2005).
J. L. Smialek, Surface and Interface Analysis31, 582 (2001).
J. L. Smialek, Metallurgical Transactions A22, 739 (1991).
D. R. Sigler, Oxidation of Metals32, 337 (1989).
D. J. Young, D. Naumenko, L. Niewolak, E. Wessel, L. Singheiser and W. J. Quadakkers, Materials and Corrosion61, 838 (2010).
C. Geers and I. Panas, Oxidation of Metals91, 55 (2019).
V. Babic, C. Geers, B. Jönsson and I. Panas, Electrocatalysis8, 565 (2017).
R. Swadźba, L. Swadźba, J. Wiedermann, M. Hetmańczyk and B. Witala, Oxidation of Metals82, 195 (2014).
Y. Chen, R. C. Reed and E. A. Marquis, Oxidation of Metals82, 457 (2014).
K. A. Unocic and B. A. Pint, Surface and Coatings Technology237, 8 (2013).
K. A. Unocic, E. Essuman, S. Dryepondt and B. A. Pint, Materials at High Temperatures29, 171 (2012).
V. Kochubey, H. Al-Badairy, G. Tatlock, J. Le-Coze, D. Naumenko and W. J. Quadakkers, Materials and Corrosion56, 848 (2005).
D. Naumenko, V. Kochubey, J. Le-Coze, E. Wessel, L. Singheiser and W. J. Quadakkers, Materials Science Forum461–464, 489 (2004).
B. A. Pint, K. L. More and I. G. Wright, Materials at High Temperatures20, 375 (2003).
P. Y. Hou, Journal of the American Ceramic Society86, 660 (2003).
B. A. Pint, A. J. Garratt-Reed and L. W. Hobbs, Journal of the American Ceramic Society81, 305 (1998).
K. A. Unocic, Y. Chen, D. Shin, B. A. Pint and E. A. Marquis, Micron109, 41 (2018).
V. K. Tolpygo and D. R. Clarke, Materials at High Temperatures20, 261 (2003).
B. A. Pint and K. B. Alexander, Journal of The Electrochemical Society145, 1819 (1998).
B. A. Pint, Oxidation of Metals45, 1 (1996).
B. A. Pint, J. R. Martin and L. W. Hobbs, Oxidation of Metals39, 167 (1993).
A. H. Heuer, D. B. Hovis, J. L. Smialek and B. Gleeson, Journal of the American Ceramic Society94, 146 (2011).
A. H. Heuer, T. Nakagawa, M. Z. Azar, et al., Acta Materialia61, 6670 (2013).
H. Guhl, H.-S. Lee, P. Tangney, et al., Acta Materialia99, 16 (2015).
A. H. Heuer, M. Zahiri Azar, H. Guhl, et al., Journal of the American Ceramic Society99, 733 (2016).
B. A. Pint, Journal of the American Ceramic Society86, 686 (2003).
P. Kofstad, A. Rahmel, R. A. Rapp and D. L. Douglas, Oxidation of Metals32, 125 (1989).
P. Kofstad, High Temperature Corrosion, (Elsevier, Amsterdam, 1988).
N. Mortazavi, C. Geers, M. Esmaily, et al., Nature Materials17, 610 (2018).
H. Yu, S. Ukai, S. Hayashi and N. H. Oono, Corrosion Science127, 147 (2017).
MS. Materials Studio 6.0 Accelry Inc.
H. J. Monkhorst and J. D. Pack, Physical Review B13, 5188 (1976).
S. Grimme, J. Antony, S. Ehrlich and H. Krieg, The Journal of Chemical Physics132, 154104 (2010).
D. L. Ram, G. J. Tatlock and U. Falke, Materials at High Temperatures22, 497 (2005).
J. T. Ellingham, Journal of the Society of Chemical Industry63, 125 (1944).
G. Brillant, Progress in Nuclear Energy53, 125 (2011).
Y. Yamamoto, B. A. Pint, K. A. Terrani, K. G. Field, Y. Yang and L. L. Snead, Journal of Nuclear Materials467, 703 (2015).
Acknowledgements
Open access funding provided by Chalmers University of Technology. This work was carried out partly within the Swedish High Temperature Corrosion Center (CG and VB) and within the Swedish Foundation for Strategic Research project SAFETY (VB and IP). Computations were performed on resources at Chalmers Centre for Computational Science and Engineering (C3SE) provided by the Swedish National Infrastructure for Computing (SNIC).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Babic, V., Geers, C. & Panas, I. Reactive Element Effects in High-Temperature Alloys Disentangled. Oxid Met 93, 229–245 (2020). https://doi.org/10.1007/s11085-019-09946-6
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11085-019-09946-6