Abstract
Chitosan is a natural biopolymer that is classified among the most important biodegradable polysaccharides widely used in different environmental and industrial applications, such as tissue engineering, biomedical devices, electronics and supercapacitors, water filtration, and food packaging. Theoretical infrared spectra of chitosan were computed using both Hartree–Fock (HF) and Density Functional Theory (DFT) methods, with different basis sets, including 3-21g, 6-31g, 6-311g, LANL2DZ, and LANL2MB, to identify the ideal basis set that is closest to the experimental results. DFT:B3LYP/3-21g** was the best model for chitosan and was used to investigate its functionalization with various functional groups such as (OH, NH2, COOH, CH3, CHO, CN, SH) and graphene oxide (GO). Molecular electrostatic potential, total dipole moment, and HOMO–LUMO band gap (∆E) calculations indicated that Chitosan-GO is the most reactive and stable structure, with a ∆E of 0.3023 eV. Consequently, Chitosan–GO composite was prepared and analyzed using ATR–FTIR spectroscopy. The spectra revealed a new band at 1620 cm−1, which was attributed to the COOH group of GO and was red-shifted owing to the hydrogen bonding between the GO and NH2 of chitosan, confirming the synthesis of Chitosan–GO composite. The significant improvement in the electronic properties of Chitosan-GO based on the obtained results promotes it to be used in electronic applications such as the development of electrodes for supercapacitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Graphene (G) is an sp2 hybridized two–dimensional single-layer plate of carbon atoms, in which carbon atoms are organized in a hexagonal pattern (Albertsen et al. 1980; Armakovic et al. 2016). Because of the intense interactions between carbon atoms in graphene, it has a very limited water solubility. Graphene oxide (GO) is one of graphene’s derivatives, having different oxygen functional groups on its surface such as hydroxyl (–OH), carbonyl (C = O), and carboxylic (–COO) groups, allowing it to interact with other materials more effectively. These groups enable GO to form covalent connections with a wide range of substrates, particularly polymers like chitosan (Bustos-Ramírez et al. 2013; Han et al. 2011). GO is more biocompatible than G and has a wider range of applications in medicine, which include but not limited to providing very strong and light composites (Vashist and Luong 2015; Kandjou et al. 2019), drug delivery (as a drug carrier) (Ghosh et al. 2010), tissue engineering to develop high-level special scaffolding (Olad et al. 2019), and biosensors (Wang et al. 2011).
2D G or GO sheets are projected to be a potential nanoscale filler for the next generation of nanocomposite materials due to their amazing mechanical properties and affordable costs. Some notable papers have covered the potential applications of GO–polymer nanocomposites, such as organic conductive films and heat-resistant materials (Zheng et al. 2014; Wang et al. 2012). Since GO may be dispersed at the individual sheet level in water, so if water is employed as a common solvent for both GO and the polymer matrix, a fully molecular-level dispersion of GO can be achieved. Furthermore, the epoxy groups in GO have shown preference to react with primary amine groups through addition, which has been widely employed to modify GO. Therefore, in addition to the hydrogen bonding between them, a new mixture of chitosan and GO can be formed by a specific interaction (Selatile et al. 2021). GO has been commonly used in biomedical field, such as polymeric-GO composites for tissue engineering and regeneration applications (Khan et al. 2022; Al-Arjan et al. 2022; Khan et al. 2021a, b), and for drug delivery (Khan et al. 2021b, c; Al-Arjan et al. 2020). GO has also proven to be a very promising and efficient material for supercapacitors applications (Tamang et al. 2023). To enhance the capacitance of reduced GO (rGO) for supercapacitor applications, it was coupled to amino hydroquinone dimethylether (Luo et al. 2023). GO was also introduced to enhance the performance of nickel oxide supercapacitor electrodes (BinSabt et al. 2023). rGO was also utilized to synthesize a novel high–performance charge storage Al2O3-rGO hybrid electrode (Ratha et al. 2023). A GO–Thioamide hybrid composite was developed as a high–performance supercapacitor electrode (Czepa et al. 2023). A GO/Co3O4 nanocomposite was also synthesized and presented as a highly efficient material for supercapacitor application (Veeresh et al. 2022).
Chitosan is a biodegradable natural biopolymer (Yang et al. 2007) that has emerged as a popular choice among scientists for research and development, as well as for environmental protection through the production of biodegradable products (Singh et al. 2013; Islam et al. 2013, 2012). Green biopolymer-based composites have a variety of important advantages, including being biodegradable, renewable, environmentally friendly, and long-lasting.
As a green biopolymer, chitosan is widely used in membranes, tissue engineering, drug delivery, wound dressing, and packaging materials owing to its high biocompatibility, antibacterial properties, biodegradability, and non-toxicity (Bansal et al. 2011; Antony et al. 2019; Ueno 2001; Morin-Crini et al. 2019). It is derived from chitin, the second most abundant natural biopolymer on earth after cellulose, and it has gained a lot of attention because of its low cost, low immunogenicity, and the fact that it is a natural green adsorbent with hydroxyl, amino, and carbonyl groups (Li et al. 2020). This cationic amino polysaccharide has multiple reactive sites for grafting, ionic contacts, insoluble ionic complexes with a variety of water-soluble anionic polymers, and metal ion adsorption (Saheed et al. 2021). Furthermore, chitosan is a stimulus-responsive polymer whose solubility may be adjusted by altering the pH value (Antony et al. 2019). The molecular weight and degree of deacetylation (DDA) of chitosan influence its unique features. Re-acetylation can diminish DDA, while acidic de-polymerization can reduce molecular weight (Desai et al. 2021). To enhance the mechanical properties of chitosan, different approaches such as nanoparticles inclusion, crosslinking, graft copolymerization, complexation, chemical changes, and mixing are used (de Oliveira et al. 2021; Shoueir et al. 2021). Using nano-fillers such as carbon nanotubes, clays, and other materials has already proven to be a viable solution to different problems (Sanusi et al. 2020). Carbon nanotubes are the best reinforcing agents, but their industrial use is limited due to their costly production methods and poor dispersibility (Mohd Nurazzi et al. 2021). Similar to GO, chitosan has also been one of the frequently used materials in the field of supercapacitors. Supercapacitor electrodes were fabricated from activated carbon derived from chitosan biomass (Abu et al. 2023). Chitosan was also used as a binder in the fabrication of polyaniline/multi-walled carbon nanotubes supercapacitors (Yesilyurt et al. 2023). Films of carboxylated chitosan-graft-poly (Vinyl-2-Pyrrolidone) were also synthesized, and electrochemical investigation revealed their potential as electrodes in supercapacitor devices (Zaghlool et al. 2023). Carboxylated chitosan matrix was also used with polyacrylamide and glycerol to fabricate a polymeric hydrogel electrolyte for solid-state supercapacitor application (Wang et al. 2023).
In this study, we present a straightforward and environmentally friendly method for manufacturing chitosan/GO composite films (Han et al. 2011) that involves integrating GO into the chitosan matrix with diluted acetic acid as the processing solvent. Density functional theory (DFT) computations have recently been widely employed to investigate the electrical and chemical characteristics of G, GO, and functionalized G (Hernández Rosas et al. 2011; Anota et al. 2013a). Furthermore, DFT simulations were utilized to investigate chitosan and functionalized chitosan (Juárez et al. 2013; Anota et al. 2013b; Rodríguez-Juárez et al. 2015) using several models, such as the B3LYP/6-31g(d) model (Juarez-Morales et al. 2017).
The aim of this study is to obtain computed vibrational spectra comparable to experimental ones for chitosan in order to identify the most suitable basis set. To achieve this goal, computations were performed using Hartree–Fock methods (HF) and B3LYP method of DFT (DFT:B3LYP) using a variety of basis sets, including 3-21g, 6-31g, 6-311g, LANL2DZ, LANL2MB and 3-21g**. The most suitable basis set will then be utilized to determine the best site for functionalization of chitosan’s chain (center or terminal). Finally, several functional groups such as OH, NH2, COOH, CH3, CHO, CN, SH and GO are introduced and examined for the functionalization of chitosan through the optimum site of interaction in order to investigate the impact of functionalization on the electronic properties of chitosan.
2 Material and methods
2.1 Materials
Chitosan (low molecular weight; M.W. 120 kDa) was purchased from ABCO Laboratories Eng. Ltd (Gillingham, England). GO was synthesized using graphite powder (particle size < 20 µm) from Fluke, Germany, and solvents such as H2SO4 (98%), H2O2 (30%), and HCl (33%) were all acquired from El-Nasr Pharmaceutical Company, Egypt. KMnO4 98% was acquired from Alfa Aesar, Germany.
2.2 Synthesis of GO
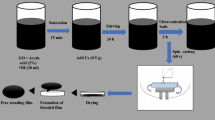
The Hummers method (Hummers 1958) was used to synthesize GO. 1 g of graphite was mixed with 35 ml of H2SO4 and 3 g of KMnO4, and then stirred for 1 h in an ice bath at temperatures 0–4 °C. 105 ml of H2O2 were carefully mixed with the solution for 1 h, and the mixture was heated up to approximately 100 °C. This mixture was then diluted with 280 ml of distilled water. Precipitated GO was washed with 2 M HCl, then with deionized water and dried.
2.3 Preparation of chitosan and chitosan-GO
For the preparation of chitosan and chitosan-GO films, 0.25 g of chitosan was dissolved in 100 ml of distilled water containing 2%v/v acetic acid with continuous stirring for 2 h at 70 °C until chitosan was completely dissolved. The solution was then doped with GO (20 wt%) and stirred again for approximately 2 h at 70 °C until a homogeneous solution was obtained. The solution was then cast in glass petri dishes and dried at room temperature for 5 days.
2.4 Fourier transform infrared spectroscopy
Attenuated total reflection (ATR) FTIR spectra were obtained using Vertex 70 FTIR spectrometer from Bruker Optik GmbH, Germany, equipped with diamond ATR crystal system in the spectral range of 4000–400 cm–1 with the resolution of 4 cm−1.
2.5 Calculation details
Energy optimization and vibrational calculations for the studied structures were conducted using both HF and DFT:B3LYP (Lieb and Simon 1977; Becke 1993; Lee et al. 1988; Vosko et al. 1980) methods, using various basis sets including 3-21g, 6-31g, 6-311g, LANL2DZ and LANL2MB. Calculations were performed with Gaussian 09 software (Frisch et al. 2010) at Molecular Spectroscopy and Modeling Unit, National Research Centre, Cairo, Egypt. In addition to vibrational spectra, the physical parameters of total dipole moment (TDM), HOMO–LUMO band gap energy (∆E), and molecular electrostatic potential (MESP) were also calculated.
3 Result and discussion
3.1 ATR-FTIR characterization of chitosan and chitosan–GO
The FTIR spectrum of chitosan is demonstrated in Fig. 1 and its characteristic FTIR bands and their assigment are demonstrated in Table 1 (Brudzyńska et al. 2023; Sulej et al. 2023; Ibrahim et al. 2011; Anandhavelu and Thambidurai 2011). The band at 3410 cm–1 is attributed to O–H coupled with N–H stretching vibrations. The band at 2920 cm–1 is assigned for C–H stretching. Carbonyl C = O (amide I) is centered at 1655 cm–1, while C–N stretching and N–H bending vibration (amide II) is centered at 1546 cm–1. The bands at 1420 and 1380 cm–1 are corresponding to CH2 and CH3 bending vibrations, respectively. C–O stretching vibrations are located at ⁓1150–1035 cm–1. Finally, the band at 895 cm–1 is ascribed to the C–N fingerprint band.
Figure 1 and Table 1 demonstrate the characteristic FTIR bands of Chitosan–GO film. A broad stretching absorption band at 3430 cm–1 is attributed to OH groups of chitosan and GO. A new band appeared at 1620 cm–1 which is assigned to the carboxyl (COOH) group of GO, which appeared at a lower wavenumber as a result of hydrogen bonding between the NH2 group of chitosan and COOH group of GO. Finally, the band representing NH bending shifted to a higher wavenumber at 1557 cm–1, which reflects the interaction between chitosan and GO via the NH2 group (Samuel et al. 2019). Results confirmed the proper formation of Chitosan–GO composite. In a previous study (Ezzat et al. 2023), we have characterized the FTIR spectrum of pristine GO.
3.2 Theoretical IR result for chitosan
Three units of chitosan were initially optimized, and their vibrational spectra were calculated using HF and DFT:B3LYP at various basis sets as shown in Tables 2 and 3, respectively. The calculated vibrational spectra were compared with the experimental spectrum. The theoretical IR findings from several theories and basis sets were analyzed to identify the ideal basis set providing the best results in good agreement with the experimental data. The characteristic IR frequencies, which provide fingerprint information, have played a very important role in many research disciplines in chemistry. Therefore, the theoretical IR frequencies of chitosan calculated at different methods and basis sets, and corrected with an ideal scaling factor were evaluated by comparing them with the experimental results. Experimental and theoretical IR results presented in Tables 1 and 2 indicated that the spectrum calculated using DFT:B3LYP/3-21gmodel had the closest values to the experimental data. Consequently, and for higher accuracy, IR frequencies utilizing 3-21g** basis set were calculated to be compared with those obtained using 3-21g basis set. Several vibrational modes resulting from DFT: B3LYP/3-21g** calculations appeared to be identical to the experimental data. This result suggested that DFT:B3LYP/3-21g** can be used to investigate chitosan functionalized by various functional groups and GO to obtain theoretical results in closer agreement with the experimental ones. It must, however, be mentioned that B3LYP/3-21g** has some limitations such as lager bond and complexations energies than experiment for loosely bound systems, in addition to poor prediction of ionization potentials; however, despite these limitations, the B3LYP/3-21g** is quite sufficient for small molecular systems, such as chitosan, and can successfully predict the geometry and electronic structure of such systems (Riley et al. 2007; Zandler and D’Souza 2006).
It is, therefore, worth mentioning that functionlaized chitosan was also subjected to vibrational frequency caculations. Figure 2 presents some examples of the DFT:B3LYP/3-21g** calculated IR spectra for functionalized chitosan. The calculated IR spectra demonstrated positive frequencies which is an indication that the calculated frequencies are corresponding to energy minimum, which is a good indicator for the validity of the studied structures at this level of theory.
3.3 Binding energy
Based on the theoretical IR results, the DFT:B3LYP/3-21g** was selected as the best model to study functionalized chitosan and to identify the optimal site for interaction (center or terminal). As shown in Fig. 3, chitosan was proposed to be functionalized with OH group once through a central unit and once through a terminal unit. Since chitosan’s functionalization takes place through the active site of NH2 group, the model hypothesized a complex interaction between the OH group and the active site of chitosan. The binding energy of the two proposed interactions was obtained in order to evaluate the most probable place of connection.
The binding energies of chitosan functionalized with OH group through the two proposed site of interactions were calculated twice. The calculated total energy and binding energies are presented in Table 4. From the obtained data, Chitosan-OH-Center was found to have a larger positive binding energy value than that of Chitosan-OH-Terminal. Consequently, Chitosan-OH-Center had the lowest negative binding energy and is then the most suitable site for chitosan’s functionalization (Tavakol 2017).
3.4 Total dipole moment, HOMO–LUMO band gap and molecular electrostatic potential of functionalized chitosan
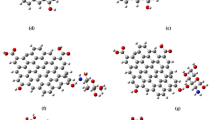
As shown in Fig. 4, OH, NH2, COOH, CH3, CHO, CN, SH and GO as functional groups were used to functionalize chitosan via the perfect site of chitosan’s functionalization identified by binding energy calculations. TDM, ∆E, and MESP were calculated for chitosan models with the specified functional groups and GO to evaluate the influence of functionalization on chitosan’s reactivity. Increased TDM together with decreased ∆E is considered a significant contributor demonstrating the enhancement of electronic properties and structural stability (Al-Fifi et al. 2014; Al-Bagawi et al. 2020). Table 5 demonstrates the variance in calculated ∆E and TDM values of all proposed models. TDM of functionalized chitosan increased from 4.071 Debye to 5.724, 5.245, 6.736, 5.207, 6.957, 8.701, 6.957 and 50.836 Debye for chitosan functionalized with OH, NH2, COOH, CH3, CHO, CN, SH and GO, respectively. On the other hand, ∆E of all functionalized chitosan structures decreased from 7.919 eV to 6.860, 7.183, 7.003, 5.207, 6.571, 7.571, 6.571 and 0.3023 eV for chitosan functionalized with OH, NH2, COOH, CH3, CHO, CN, SH and GO, respectively. These results clearly indicate that Chitosan-GO have the highest reactivity, owing to its highest TDM and lowest ∆E values, which also dedicate it to be the most stable structure.
Optimized structures and MESP of chitosan interacted with different functional groups through center as (a) Chitosan; (b) Chitosan-OH; (c) Chitosan-NH2; (d) Chitosan-COOH; (e) Chitosan-CH3; (f) Chitosan-CHO; (g) Chitosan-CN; (h) Chitosan-SH; and (i) Chitosan-GO calculated using DFT:B3LYP/3-21 g** model
MESP is also an important descriptor for studying the reactivity of chemical interactions by mapping the electrostatic potential of a given structure based on the electron density formed by the nuclei and electrons in that structure (Ourhzif et al. 2021; Donglai et al. 2007). The MESP values are displayed as a color map on the molecule’s surface, with the colors arrayed from highest electron density area (red color) to lowest electron density one (blue color) (Ourhzif et al. 2021; Donglai et al. 2007; Mol et al. 2022). MESP colored maps of chitosan and functionalized chitosan with the different groups are illustrated in Fig. 4. The resulting maps confirmed that the NH2 group of chitosan is the most reactive site of chitosan molecule. The intensity of the red-colored areas on the polymer chain increased when chitosan was functionalized with different functional groups. All functional groups exerted a physical characteristic change on chitosan’s electronic properties and reactivity, especially Chitosan–GO which demonstrated significant improvement of the electronic properties and can, in turn, be used in electronic applications such as the development of electrodes for supercapacitors.
4 Conclusion
Experimental FTIR and molecular modeling calculations with different levels of theory and basis sets were conducted for chitosan and chitosan-GO composite. Experimental FTIR spectra of chitosan and chitosan-GO film verified the formation of chitosan-GO composite, and confirmed that the interaction is taking place through NH2 group of chitosan. The calculation of IR spectrum at both HF and DFT levels with different basis sets revealed that DFT:B3LYP/3-21g** is the best model, yielding several vibrational modes identical to the experimental FTIR data. Binding energy calculations for chitosan confirmed that the most probable site for chitosan’s interaction is through center units. DFT:B3LYP/3-21g** model was used to study the effect of the functionalization of chitosan by OH, NH2, COOH, CH3, CHO, CN, SH and GO functional groups, and the results confirmed that Chitosan-GO is the most reactive and stable structure based on the obtained TDM, ΔE and MESP values. The significant enhancement in the electronic properties of Chitosan-GO composite can promote it for potential electronic applications such as supercapacitor electrodes. Finally, it can also be concluded that molecular modeling is a suitable and very useful technique in studying both electronic and vibrational properties of raw and functionalized biopolymers such as chitosan.
Availability of data and materials
The data are available from the corresponding author upon request. Contact Medhat A. Ibrahim (medahmed6@yahoo.com).
References
Abu, S.M., Ansari, M.N.M., Al-Shetwi, A.Q., Muttaqi, K.M., Hannan, M.A.: The performance of chitosan-based activated carbon for supercapacitor applications towards sustainable energy technologies. IEEE Trans. Ind. Appl. 59(3), 3133–3141 (2023). https://doi.org/10.1109/TIA.2023.3255215
Al-Arjan, W.S., Aslam Khan, M.U., Nazir, S., Abd Razak, S.I., Abdul Kadir, M.R.: Development of arabinoxylan-reinforced apple pectin/graphene oxide/nano-hydroxyapatite based nanocomposite scaffolds with controlled release of drug for bone tissue engineering: in-vitro evaluation of biocompatibility and cytotoxicity against MC3T3-E1. Coatings 10, 1120 (2020). https://doi.org/10.3390/coatings10111120
Al-Arjan, W.S., Khan, M.U.A., Almutairi, H.H., Alharbi, S.M., Razak, S.I.A.: pH-responsive PVA/BC-f-GO dressing materials for burn and chronic wound healing with curcumin release kinetics. Polymers 14(10), 1949 (2022). https://doi.org/10.3390/polym14101949
Al-Bagawi, A.H., Bayoumy, A.M., Ibrahim, M.A.: Molecular modeling analyses for graphene functionalized with Fe3O4 and NiO. Heliyon 6(7), e04456 (2020). https://doi.org/10.1016/j.heliyon.2020.e04456
Albertsen, P., Jørgensen, P.L., Yeager, D.L.: Indirect nuclear spin-spin coupling constants within the coupled multiconfiguration Hartree-Fock approximation. Chem. Phys. Lett. 76, 354–358 (1980). https://doi.org/10.1016/0009-2614(80)87040-0
Al-Fifi, Z., Eid, M., Saleh, N.A., Ibrahim, M.: Molecular modelling analyses of the substituted 3′-Azido-2′, 3′ dideoxythymidine. J. Comput. Theor. Nanosci. 11, 409–412 (2014). https://doi.org/10.1166/jctn.2014.3369
Anandhavelu, S., Thambidurai, S.: Preparation of chitosan–zinc oxide complex during chitin deacetylation. Carbohydr. Polym. 83, 1565–1569 (2011). https://doi.org/10.1016/j.carbpol.2010.10.006
Anota, E.C., Juárez, A.R., Castro, M., Cocoletzi, H.H.: A density functional theory analysis for the adsorption of the amine group on graphene and boron nitride nanosheets. J. Mol. Model. 19, 321–328 (2013a). https://doi.org/10.1007/s00894-012-1539-4
Anota, E.C., Rodríguez, L.D., Cocoletzi, G.H.: Influence of point defects on the adsorption of chitosan on graphene-like BN nanosheets. Graphene. 1, 124–130 (2013b). https://doi.org/10.1166/graph.2013.1014
Antony, R., Arun, T., Manickam, S.T.D.: A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 129, 615–633 (2019). https://doi.org/10.1016/j.ijbiomac.2019.02.047
Armakovic, S., Armakovic, S.J., Vranes, M., Tot, A., Gadzuric, S.: Determination of reactive properties of 1-butyl-3-methylimidazolium taurate ionic liquid employing DFT calculations. J. Mol. Liq. 222, 796–803 (2016). https://doi.org/10.1016/j.molliq.2016.07.094
Bansal, V., Sharma, P.K., Sharma, N., Pal, O.P., Malviya, R.: Applications of chitosan and chitosan derivatives in drug delivery. Biol. Res. 5, 28–37 (2011)
Becke, A.D.: Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 98, 5648–5652 (1993). https://doi.org/10.1063/1.464913
BinSabt, M.H., Galal, A., Abdel Nazeer, A.: Enhancement of supercapacitor performance of electrochemically grown nickel oxide by graphene oxide. Materials (Basel, Switzerland) 16(8), 3068 (2023). https://doi.org/10.3390/ma16083068
Brudzyńska, P., Sionkowska, A., Grisel, M.: Silk textiles dyeing by plant-derived colorant in the presence of chitosan and shellac. Fibers Polym. 24, 2761–2771 (2023). https://doi.org/10.1007/s12221-023-00250-4
Bustos-Ramírez, K., Martínez-Hernández, A.L., Martínez-Barrera, G., Icaza, M.D., Castaño, V.M., Velasco-Santos, C.: Covalently bonded chitosan on graphene oxide via redox reaction. Materials 6(3), 911–926 (2013). https://doi.org/10.3390/ma603091
Czepa, W., Witomska, S., Samorì, P., Ciesielski, A.: A graphene oxide-thioamide polymer hybrid for high-performance supercapacitor electrodes. Small Sci. 3, 2300013 (2023). https://doi.org/10.1002/smsc.202300013
Desai, R., Pachpore, R., Patil, A., Jain, R., Dandekar, P.: Review of the structure of chitosan in the context of other sugar-based polymers. In: Jayakumar, R., Prabaharan, M. (eds.) Chitosan for Biomaterials III. Advances in Polymer Science, vol. 287, pp. 23–74. Springer, Cham (2021). https://doi.org/10.1007/12_2021_89
Donglai, W., Hongtao, S., Yuchun, Z.: Theoretical study on molecular electrostatic potential of C78. J. Rare Earths 25(2), 210–214 (2007). https://doi.org/10.1016/S1002-0721(07)60075-1
Ezzat, H.A., Hegazy, M.A., Nada, N.A., Osman, O., Ibrahim, M.A.: Studying the optical and thermal properties of Cs/ZnO and Cs/ZnO/GO hybrid nanocomposites. Opt. Mater. 135, 113244 (2023). https://doi.org/10.1016/j.optmat.2022.113244
Frisch, M.J., et al.: Gaussian 09, Revision C.01, Gaussian, Inc., Wallingford (2010).
Ghosh, D., Chandra, S., Chakraborty, A., Ghosh, S.K., Pramanik, P.A.: Novel graphene oxide-para amino benzoic acid nanosheet as effective drug delivery system to treat drug resistant bacteria. Int. J. Pharm. Sci. Drug. Res. 2, 127–133 (2010)
Han, D., Yan, L., Chen, W., Li, W.: Preparation of chitosan/graphene oxide composite film with enhanced mechanical strength in the wet state. Carbohydr. Polym. 83, 653–658 (2011a). https://doi.org/10.1016/j.carbpol.2010.08.038
Hernández Rosas, J.J., Ramírez Gutiérrez, R.E., Escobedo-Morales, A., Chigo Anota, E.: First principles calculations of the electronic and chemical properties of graphene, graphane, and graphene oxide. J. Mol. Model. 17, 1133–1139 (2011). https://doi.org/10.1007/s00894-010-0818-1
Hummers, W.S., Jr.: Preparation of graphitic oxide. J. Am. Chem. Soc. 80, 1339 (1958). https://doi.org/10.1021/ja01539a017
Ibrahim, M., Osman, O., Mahmoud, A.A.: Spectroscopic analyses of cellulose and chitosan: FTIR and modeling approach. J. Comput. Theor. Nanosci. 8, 117–123 (2011). https://doi.org/10.1166/jctn.2011.1668
Islam, A., Yasin, T., Bano, I., Riaz, M.: Controlled release of aspirin from pH-sensitive chitosan/poly(vinyl alcohol) hydrogel. J. Appl. Polym. Sci. 124, 4184–4192 (2012). https://doi.org/10.1002/app.35392
Islam, A., Riaz, M., Yasin, T.: Structural and viscoelastic properties of chitosan-based hydrogel and its drug delivery application. Int. J. Biol. Macromol. 59, 119–124 (2013). https://doi.org/10.1016/j.ijbiomac.2013.04.044
Juárez, A.R., Anota, E.C., Cocoletzi, H.H., Riveros, A.F.: Adsorption of chitosan on BN nanotubes: a DFT investigation. Appl. Surf. Sci. 268, 259–264 (2013). https://doi.org/10.1016/j.apsusc.2012.12.075
Juarez-Morales, L.A., Hernandez-Cocoletzi, H., Chigo-Anota, E., Aguila-Almanza, E., Tenorio-Arvide, M.G.: Chitosan-aflatoxins B1, M1 interaction: a computational approach. Curr. Org. Chem. 21, 2877–2883 (2017). https://doi.org/10.2174/1385272821666170511165159
Kandjou, V., Perez-Mas, A.M., Acevedo, B., Hernaez, M., Mayes, A.G., Melendi-Espina, S.: Enhanced covalent P-phenylenediamine crosslinked graphene oxide membranes: towards superior contaminant removal from wastewaters and improved membrane reusability. J. Hazard. Mater. 380, 120840 (2019). https://doi.org/10.1016/j.jhazmat.2019.120840
Khan, M.U.A., Al-Arjan, W.S., Binkadem, M.S., Mehboob, H., Haider, A., Raza, M.A., Abd Razak, S.I., Hasan, A., Amin, R.: Development of biopolymeric hybrid scaffold-based on AAc/GO/nHAp/TiO2 nanocomposite for bone tissue engineering: in-vitro analysis. Nanomaterials (Basel, Switzerland) 11(5), 1319 (2021a). https://doi.org/10.3390/nano11051319
Khan, M.U.A., Haider, S., Raza, M.A., Shah, S.A., Abd Razak, S.I., Abdul Kadir, M.R., Subhan, F., Haider, A.: Smart and pH-sensitive rGO/Arabinoxylan/chitosan composite for wound dressing: In-vitro drug delivery, antibacterial activity, and biological activities. Int. J. Biol. Macromol. 192, 820–831 (2021b). https://doi.org/10.1016/j.ijbiomac.2021.10.033
Khan, M.U.A., Yaqoob, Z., Ansari, M.N.M., Razak, S.I.A., Raza, M.A., Sajjad, A., Haider, S., Busra, F.M.: Chitosan/poly vinyl alcohol/graphene oxide based ph-responsive composite hydrogel films: drug release anti-microbial and cell viability studies. Polymers 13, 3124 (2021c). https://doi.org/10.3390/polym13183124
Khan, M.U.A., Razak, S.I.A., Haider, S., Mannan, H.A., Hussain, J., Hasan, A.: Sodium alginate-f-GO composite hydrogels for tissue regeneration and antitumor applications. Int. J. Biol. Macromol. 208, 475–485 (2022). https://doi.org/10.1016/j.ijbiomac.2022.03.091
Lee, C., Yang, W., Parr, R.G.: Development of the Colic-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 37, 785 (1988). https://doi.org/10.1103/physrevb.37.785
Li, B., Elango, J., Wu, W.: Recent advancement of molecular structure and biomaterial function of chitosan from marine organisms for pharmaceutical and nutraceutical application. Appl. Sci. 10, 4719 (2020). https://doi.org/10.3390/app10144719
Lieb, E.H., Simon, B.: The Hartree-Fock theory for coulomb systems. Commun. Math. Phys. 53, 185–194 (1977). https://doi.org/10.1007/BF01609845
Luo, Y., Lin, H., Chu, Y., Wang, J., Liu, N., Dong, L., Zhao, F.-G., Chen, Y., Shen, Y.: High-performance reduced graphene oxide supercapacitors enabled by simple amino hydroquinone dimethylether. Chem. Commun. 59, 7208–7211 (2023). https://doi.org/10.1039/D3CC01597A
Mohd Nurazzi, N., Asyraf, M.R.M., Khalina, A., Abdullah, N., Sabaruddin, F.A., Kamarudin, S.H., Ahmad, S., Mahat, A.M., Lee, C.L., Aisyah, H.A., et al.: Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: an overview. Polymers 13, 1047 (2021). https://doi.org/10.3390/polym13071047
Mol, G.P.S., Aruldhas, D., Joe, I.H.: Chemical reactivity, molecular electrostatic potential and in-silico analysis on benzimidazole fungicide benomyl. Heliyon 8, e11417 (2022). https://doi.org/10.1016/j.heliyon.2022.e11417
Morin-Crini, N., Lichtfouse, E., Torri, G., Crini, G.: Fundamentals and applications of chitosan. In: Crini, G., Lichtfouse, E. (eds.) Sustainable Agriculture Reviews 35. Sustainable Agriculture Reviews, vol. 35, pp. 49–123. Springer, Cham (2019). https://doi.org/10.1007/978-3-030-16538-3_2
Olad, A., Bakht Khosh Hagh, H.: Graphene oxide and amin-modified graphene oxide incorporated chitosan gelatin scaffolds as promising materials for tissue engineering. Compos. B. Eng. 162, 692–702 (2019). https://doi.org/10.1016/j.compositesb.2019.01.040
de Oliveira, A.L.B., et al.: Chitosan nanoparticle: alternative for sustainable agriculture. In: Nascimento, R.F.d., Neto, V.d.O.S., Fechine, P.B.A., Freire, P.d.T.C. (eds.) Nanomaterials and Nanotechnology. Materials Horizons: From Nature to Nanomaterials, pp. 95–132. Springer, Singapore (2021). https://doi.org/10.1007/978-981-33-6056-3_4
Ourhzif, E., Ketatni, E., Akssira, M., Troin, Y., Khouili, M.: Crystal structure, Hirshfeld surface analysis and DFT studies of Euphorbioside monohydrate a major bisnorsesquiterpene isolated from Euphorbia resinifera latex. J. Mol. Struct. 1241, 130511 (2021). https://doi.org/10.1016/j.molstruc.2021.130511
Ratha, S., Sahoo, S., Mane, P., Polai, B., Sathpathy, B., Chakraborty, B., Nayak, S.K.: Experimental and computational investigation on the charge storage performance of a novel Al2O3-reduced graphene oxide hybrid electrode. Sci. Rep. 13, 5283 (2023). https://doi.org/10.1038/s41598-022-23574-2
Riley, K.E., Op’t Holt, B.T., Merz, K.M., Jr.: Critical assessment of the performance of density functional methods for several atomic and molecular properties. J. Chem. Theory Comput. 3(2), 407–433 (2007). https://doi.org/10.1021/ct600185a
Rodríguez-Juárez, A., Hernández-Cocoletzi, H., Chigo-Anota, E.: Influencia de los defectos puntuales sobre las propiedades estructurales y electrónicas de nanotubos de BN funcionalizados con quitosano. Rev. Mex. Ing. Quim. 14, 789–799 (2015)
Saheed, I.O., Da Oh, W., Suah, F.B.M.: Chitosan modifications for adsorption of pollutants–a review. J. Hazard. Mater. 408, 124889 (2021). https://doi.org/10.1016/j.jhazmat.2020.124889
Samuel, M.S., Bhattacharya, J., Raj, S., Santhanam, N., Singh, H., Singh, N.P.: Efficient removal of Chromium (VI) from aqueous solution using chitosan grafted graphene oxide (CS-GO) nanocomposite. Int. J. Biol. Macromol. 121, 285–292 (2019). https://doi.org/10.1016/j.ijbiomac.2018.09.170
Sanusi, O.M., Benelfellah, A., Hocine, N.A.: Clays and carbon nanotubes as hybrid nanofillers in thermoplastic-based nanocomposites – a review. Appl. Clay Sci. 185, 105408 (2020). https://doi.org/10.1016/j.clay.2019.105408
Selatile, K., Ray, S.S., Ojijo, V., Sadiku, R.E.: Morphological, thermal, and mechanical properties of electrospun recycled poly(ethylene terephthalate)/graphene oxide composite nanofiber membranes. ACS Omega 6, 21005–21015 (2021). https://doi.org/10.1021/acsomega.1c02578
Shoueir, K.R., El-Desouky, N., Rashad, M.M., Ahmed, M.K., Janowska, I., El-Kemary, M.: Chitosan based-nanoparticles and nanocapsules: Overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 167, 1176–1197 (2021). https://doi.org/10.1016/j.ijbiomac.2020.11.072
Singh, A., Sinsinbar, G., Choudhary, M., Kumar, V., Pasricha, R., Verma, H.N., Singh, S.P., Arora, K.: Graphene oxide-chitosan nanocomposite based electrochemical DNA biosensor for detection of typhoid. Sens. Actuat. B Chem. 185, 675–684 (2013). https://doi.org/10.1016/j.snb.2013.05.014
Sulej, J., Osińska-Jaroszuk, M., Jaszek, M., Olszewska, A., Belcarz, A., Piątek-Gołda, W.: Chitosan as a promising support of a CDH activity preservation system for biomedical and industrial applications. Int. J. Mol. Sci. 24, 4535 (2023). https://doi.org/10.3390/ijms24054535
Tamang, S., Rai, S., Bhujel, R., Bhattacharyya, N.K., Swain, B.P., Biswas, J.: A concise review on GO, rGO and metal oxide/rGO composites: fabrication and their supercapacitor and catalytic applications. J. Alloy. Compd. 947, 169588 (2023). https://doi.org/10.1016/j.jallcom.2023.169588
Tavakol, H.: Study of binding energies using DFT methods, vibrational frequencies and solvent effects in the interaction of silver ions with uracil tautomers. Arab. J. Chem. 10, S786–S799 (2017). https://doi.org/10.1016/j.arabjc.2012.12.007
Ueno, H.: Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 52, 105–115 (2001). https://doi.org/10.1016/s0169-409x(01)00189-2
Vashist, S.K., Luong, J.H.T.: Recent advances in electrochemical biosensing schemes using graphene and graphene-based nanocomposites. Carbon 84, 519–550 (2015). https://doi.org/10.1016/j.carbon.2014.12.052
Veeresh, S., Ganesha, H., Nagaraju, Y.S., Vijeth, H., Vandana, M., Basappa, M., Devendrappa, H.: Graphene oxide/cobalt oxide nanocomposite for high-performance electrode for supercapacitor application. J. Energy Storage 52, 104715 (2022). https://doi.org/10.1016/j.est.2022.104715
Vosko, S.H., Wilk, L., Nusair, M.: Accurate spin-dependent electron liquid correlation energies for local spin density calculations: a critical analysis. Can. J. Phys. 58, 1200–1211 (1980). https://doi.org/10.1139/p80-159
Wang, Y., Li, Z., Wang, J., Li, J., Lin, Y.: Graphene and graphene oxide: biofunctionalization and applications in biotechnology. Trends Biotechnol. 29, 205–212 (2011). https://doi.org/10.1016/j.tibtech.2011.01.008
Wang, C., Feng, L., Yang, H., Xin, G., Li, W., Zheng, J., Tian, W., Li, X.: Graphene oxide stabilized polyethylene glycol for heat storage. Phys. Chem. Chem. Phys. 14, 13233–13238 (2012). https://doi.org/10.1039/C2CP41988B
Wang, X., Zhang, Q., Zhao, L., Hadi, M.K., Sambasivam, S., Zhou, Q., Ran, F.: A renewable hydrogel electrolyte membrane prepared by carboxylated chitosan and polyacrylamide for solid-state supercapacitors with wide working temperature range. J. Power Sources 560, 232704 (2023). https://doi.org/10.1016/j.jpowsour.2023.232704
Yang, X., Tu, Y., Li, L., Shang, S., Tao, X.-M.: Rheological characterization of a novel thermosensitive chitosan/poly(vinyl alcohol) blend hydrogel. Carbohydr. Polym. 67, 491–499 (2007). https://doi.org/10.1016/j.carbpol.2006.06.015
Yesilyurt, E.I., Pionteck, J., Simon, F., Voit, B.: Fabrication of PANI/MWCNT supercapacitors based on a chitosan binder and aqueous electrolyte for enhanced energy storage. RSC Appl. Polym. 1, 97–110 (2023). https://doi.org/10.1039/D3LP00061C
Zaghlool, R.A., Ali, H.E., Awadallah – F, A., Aboulfotouh, M.E.: Electrochemical study of carboxylated chitosan-graft-poly (Vinyl-2-Pyrrolidone) films for supercapacitor applications. Polym.-Plast. Technol. Mater. 62(18), 2450–2467 (2023). https://doi.org/10.1080/25740881.2023.2263077
Zandler, M.E., D’Souza, F.: The remarkable ability of B3LYP/3-21G(*) calculations to describe geometry, spectral and electrochemical properties of molecular and supramolecular porphyrin–fullerene conjugates. CR Chim. 9(7–8), 960–981 (2006). https://doi.org/10.1016/j.crci.2005.12.008
Zheng, Q., Li, Z., Yang, J., Kim, J.K.: Graphene oxide-based transparent conductive films. Prog. Mater. Sci. 64, 200–247 (2014). https://doi.org/10.1016/j.pmatsci.2014.03.004
Acknowledgements
This work was carried out during the Fifth Spectroscopy Winter School, February 2023, Cairo, Egypt.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
Authors equally contributed to this work.
Corresponding author
Ethics declarations
Ethical approval
This work is not applicable for both human and/or animal studies.
Competing interests
I declare that the authors have no competing interests as defined by Springer, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elhaes, H., Ezzat, H.A., Ibrahim, A. et al. Spectroscopic, Hartree–Fock and DFT study of the molecular structure and electronic properties of functionalized chitosan and chitosan-graphene oxide for electronic applications. Opt Quant Electron 56, 458 (2024). https://doi.org/10.1007/s11082-023-05978-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-023-05978-0