Abstract
In this paper we present results of ellipsometric studies of thin organic poly-3hexylthiophene–(P3HT) and poly-3octylthiophene (P3OT) films performed in temperature range 20–300 °C. The optical dispersion spectra of refractive and extinction indices of presented organic films was determined in wavelength range 190–1700 nm for temperatures within in a given range. Also thermo-ellipsometric investigations allowed to find temperature dependence on film thickness. The determination of thermal changes of thickness and refractive index allowed us to calculate thermo-optical coefficients (TOC) for P3HT and P3OT layers for many wavelengths in measured spectral range. The obtained values of thermo-optical coefficients of thin P3HT layers, determined from combined ellipsometric and spectro-photometric investigations, are negative in visible part of spectral range of the order of 10−4 [1/K] .
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Conductive polymers such as polythiophenes have good thermal stability, chemical and absorption of radiation from the ultraviolet range and the visible region (Handbook of Luminescence 2003; Kalinowski 2005; Jaglarz et al. 2004). The distance between the HOMO and LUMO levels for this group of polymers is about 2 eV. The ease of polythiophenes use in optoelectronics, nonlinear optics, electronics results from their good conductivity and transparency in UV-Vis wavelength region (Sicot et al. 2000). Also polythiophenes are regarded as as donor in organic photovoltaic cells.
In our work we apply o polythiophenes as donor in organic photovoltaic cells. The crucial and especially important in optoelectronic application is their good process ability, environmental stability and electro-activity.
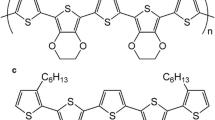
In our paper we present optical and thermooptical properties of polythiophenes whose representatives is poly (3-hexylthiophene)-P3HT (Lee et al. 2010) and and poly-3octylthiophene) P3OT (Lopez-Mata et al. 2005). The chemical structure of P3HT and P3OT are shown in Fig. 1.
Both of the studied polytiophenes have similar HOMO (5 eV for P3HT and 5.25 eV for P3OT) and LUMO levels (3 eV for P3HT and 3.35 eV for P3OT). Optical gap for P3HT is 2 eV and is bigger than for P3OT–1.8 eV.
Optical constants, namely the extinction coefficient (k) and refractive index (n), are very important parameters for understanding the mechanism of interaction between incident light and conjugated polymer films. These intrinsic properties can lead to the design of optimized solar cells with better photovoltaic performance. Despite the importance of these parameters, rather little information is available concerning the P3HTand P3OT optical constants (Kymakis and Amaratunga 2004), since many researchers focus only on absorption spectrum of P3HT, neglecting strong bulk and surface scattering losses. The refractive index n(T) of an optical medium at a given temperature T is also an important parameter for characterizing the material as derivative of n upon the temperature. To facilitate analysis of experimental results the thermo-optical coefficient (TOC) (Kang et al. 2003) is defined. The TOC is derivative of refractive index upon the temperature dn/dT. The temperature dependence of refractive indices of polymeric materials is described using the. TOC values of organic materials are more than ten times larger than those of inorganic glasses, applications of polymer optical materials to thermo-optical switches and passive optical filters at variable temperatures attract much attention (Houbertz et al. 2003). The knowledge of TOC may be essential in the analysis of dense molecular packing, controlling structural isomerism and chemical modification of precursors and in the study of polarizability changes in molecular chains in conductive polymer layers.
Thin organic conductive films optical constants can be described by using Kramers Kroning relation in wide spectral region. The K–K relations are mathematical formulas which connect the real and imaginary parts of refractive index complex function and have become basic tools in the investigation of the optical properties of materials (Lucarini et al. 2005).
According to Prod’s Homme 1960 theory, the factors that influence on TOC value are density and electronic polarizability which change with temperature
where α is linear thermal expansion coefficient and \(\varPhi\) is the temperature coefficient of the electronic polarizability, defined as: \(\varPhi = P^{ - 1} *dP/dT\)-where P is mean polarizability.
If the electronic polarizability term is dominant, the refractive index increases with temperature. On the other hand, TOC is negative when the thermal expansion coefficient is dominant. However, in the case of optical polymers, dn/dT exclusively depends on thermal expansion term because 3α is always much higher than \(\varPhi\).
2 Experimental details
In work was used P3HT solution synthesized by Sigma Aldrich.Footnote 1 The P3HT and P3OT solid layers have been deposited by a spin cast method onto crystalline silicon substrates using spin Coater Model SCV-15 (LOT-Oriel GmbH) with aligned rotational speed 2000 rotations per minute. For better accuracy, these P3HT/Si and P3OT/Si layered systems in optical and thermo-optical measurements have been considered. The use of crystalline silicon as a substrate due to the fact that its thermo-optical properties are well defined in a wide range of temperature (Complete Easy Data Analysis Manual et al. 2008). Also because the optical difference on layer –Si interface was large (at \(\lambda = 633\,{\text{nm}}\), n P3HT/P3OT < 1.9 for polytiophene films, while n Si = 3.81, for Si substrate) which increased the measurement sensitivity.
We determined spectral dependence of refractive indices in wavelength range from 300 to 1700 nm in room temperature (295 K) films thickness using the K–K relations. For this purpose the ellipsometric measurements have been performed by spectroscopic M-2000 ellipsometer (Woollam Co. Inc.).
The samples have been measured for four angles of incidence namely: 55º, 60º, 65º, 70º. To analyze the data, we have combined all angular spectra and we have fitted all data simultaneously. The data have been analyzed using Complete EASE 5.2 software.
For the determination of the P3HT and P3OT layer thickness, refractive index n and extinction k coefficients the spectroscopic ellipsometry was used (Azzam and Bashara 1995). In this technique, changes of the light polarization due to its reflection at a surface are measured. The experimentally recorded data are \(\Psi\) and ∆, which are angles defining the ratio of the amplitude Fresnel reflection coefficients r p and r s components parallel and perpendicular to incidence plane of the light.
The angle ∆ is a phase shift between the both waves.
The spectroscopic ellipsometer M-2000 was equipped with a temperature chamber holding optical.
As can be appreciated the strong absorption in the layers of P3HT and P3OT occurs in the area of 400–600 nm, with a pronounced maximum. It is also an area of abnormal dispersion of the studied layers. It can be seen when the temperature rise of the broad peak is moving maxima dispersion dependence \(n(\lambda )\) and \(k(\lambda )\) towards shorter wavelengths. The values of the refractive index of P3HT film is larger than for P3OT layer. This is due to their greater polarizability associated with the polymer chains (Jaglarz et al. 2007) which increases the optical constants. The spectral dispersion of n determined for polythiophene films look similar for many polymer layers used in optoelectronic devices.
For high temperatures higher than 250 °C the optical constants increase. It seems that this is related to the release of the hydrogen atoms of the layer and the consequent increasing of molar mass of the layer (Fig. 2).
For quality assessment of sample during annealing we control depolarization degree of light reflected from studied layers. The temperature dependence of depolarization factor determined for wavelength is shown in Fig. 3.
Depolarization gives an insight into the degree of the homogeneity of the layer and predicts possible effects of its non-uniformity, including fluctuations of the thickness and microstructure (Jaglarz et al. 2015).
As may be notice depolarization coefficient slightly increases to temperature 100 °C and above this temperature it decreases fast. This is due to breaking of the polymer chains which increases the amorphousness layers.
The temperature dependences of n and k values at λ = 633 nm are given in Fig. 4a and b.
As may be notice TOC for both polytiophene films are negative up to 210 °C for P3OT and 225 °C for P3HT. Above those temperatures the refractive and extinction indices increase with temperature. Such results mean that above transition temperatures T g the polarizability factor \(\varPhi\) occurring in formula (1) begins to dominate which is due to the change of the layers structure (phase transformation). This is glass transition temperature T g of presented polymers is depending on the layer molecular weight (Ng et al. 2010). They are also shown in column 2 of Table 1.
It seems that this is related to the release of the hydrogen atoms of the layer resulting in the increase in the molar mass of the layer but fulfils the Glaxon Dale formula (Gladstone and Dale 1958). It express the relation between the refractive index and density of a layer.
From the ellipsometric data, measured at various temperatures, the temperature dependencies of the refractive indices were determined. The values of TOC obtained for the polytiophene layers are shown in column 4 of Table 1. The TOC values we determined in temperature range 25–150 °C where thermo-optical coefficients are monotonically and linearly decreasing. Such behaviour is characteristic for the materials in which temperature dependence of the optical indices is associated with thermal expansion (Kang et al. 2003).
For this range of temperature the thermo-optical coefficients, are negative and temperature independent. It is typical for most polymers.Footnote 2 There is no phase transition change was observed in temperature region (20–200 °C). Therefore the refractive index depends only on the material density according to Glaxon-Dale theory. As one may notice from Table 1 the thermo-optical coefficient for P3HT molecules with lower molar mass is smaller than for P3OT molecules which molar mass is higher mass because cross-linking reduces considerably the thermal expansion (Pearson and Stolka 1981).
From ellipsometric study we also determined thickness dependence of presented polythiophene films on temperature. It is shown in Fig. 5.
As may notice thickness of studied films grows up with temperature to values of T g . From temperature dependence of layer thickness the thermal expansion coefficients α have been calculated in temperature range 25–150 °C. Values of α have been presented in column 5 of Table 1.
Also in this work we calculated the thermal dependences of optical path lengths dS/dT for both samples at wavelength \(\lambda = 633\,{\text{nm}}\). For this purpose we applied following formula: dS/dT = dn/dT + nα. These values have been presented in column 6 of Table 1. As may notice for both layer these values are positive. This result is particularly important for photovoltaic cells design which would work in different temperatures.
3 Conclusion
The presented results of ellipsometric study of thin organic poly-3hexylthiophene -P3HT and poly-3octylthiophene-P3OT films deposited on crystalline silicon substrate. The ellipsometric measurements were performed in temperature range 20–300 °C. In this work we determined spectral dispersion relation for studied films for various temperatures. For presented polythiophene layers we calculated thermo-optical coefficient–TOC,and thermal dependence of optical path at wavelength \(\lambda = 633\,{\text{nm}}\). Also the thermal expansion coefficients for P3HT and P3OT films have been determined. The obtained values of thermo-optical coefficients of studied layers, were negative of the order of 10−4 [1/K]. The Results of thermo-optical investigations are comparable with some conductive polymer layers.
References
Azzam, R.M.A., Bashara, N.M.: Ellipsometry and Polarized Light. North-Holland, Amsterdam (1995)
Complete easy data analysis manual. In: J.A. Woolam Co. Inc., pp. 116–118 (2015). www.jawollam.com
Gladstone, H.J., Dale, J.: Phil. J: Trans. 148, 887–894 (1958)
Handbook of Luminescence. Display materials and devices, volume1 organic light emitting diodes. In: Nalwa, H., Rohwer, L. (eds.) American Scientific Publishers (2003)
Houbertz, R., Domann, G., Cronauer, C., Schmitt, A., Martin, H., Park, J.-U., Fröhlich, L., Buestrich, R., Popall, M., Streppel, U., Dannberg, P., Wächter, C., Bräuer, A.: Thin Solid Films 442, 194–200 (2003)
Jaglarz, J., Kassiba, A., Armatys, P., Pokladko, M., Gondek, E., Sanetra, J.: Mater. Sci. Pol. 22, 389–395 (2004)
Jaglarz, J., Wagner, T., Cisowski, J., Sanetra, J.: Opt. Mater. 29, 908–912 (2007)
Jaglarz, J., Jurzecka-Szymacha, M., Tkacz-Śmiech, K., Sahraoui, B.: Opt. Mater. 39, 26–33 (2015)
Kalinowski, J.: Organic light–emitting diodes principles, characteristics, and processes, pp. 273–376. Marcel Dekker, New York (2005)
Kang, E., Bae, J.Y., Bae, B.S.: J. Sol-Gel Sci. Technol. 26, 981–984 (2003)
Kymakis, Emmanuel, Amaratunga, A.J.: Synth. Met. 142, 161–167 (2004)
Lee, W.H., Chuang, S.Y., Chen, H.L., Su, W.F., Lin, C.H.: Thin Solid Films 518, 7450–7454 (2010)
Lopez-Mata, C., Nicho, M., Hu, H., Cadenas-Pliego, G.: García- Hernández. Thin Solid Films 490, 189–195 (2005)
Lucarini, V., Saarinen, J.J., Peiponen, K.E., Vartiainen, E.M.: Kramers- Kronig Relations in Optical Materials Research. Springer, Berlin Heidelberg (2005)
Ng, A., Li, C.H., Fung, M.K., Djurišić, A.B., Zapien, J.A., Chan, W.K., Cheung, K.Y., Wong, W.Y.: J. Phys. Chem. C 114, 15094–15101 (2010)
Pearson, J.M., Stolka, M.: Poly(N-vinylcarbazole). Gordon and Breach, Science Publishers, New York (1981)
Prod’homme, L.: Phys. Chem. Glasses 4, 119–122 (1960)
Sicot, L., Fiorini, C., Lorin, A., Raimond, P., Sentein, C., Nunzi, J.M.: Sol. Energy Mater. Sol. Cells 63, 49–60 (2000)
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Advanced Materials for Photonics and Electronics.
Guest Edited by Bouchta Sahraoui, Yahia Boughaleb, Kariem Arof, Anna Zawadzka.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Jaglarz, J., Nosidlak, N. & Wolska, N. Thermo-optical properties of conducted polythiophene polymer films used in electroluminescent devices. Opt Quant Electron 48, 392 (2016). https://doi.org/10.1007/s11082-016-0649-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11082-016-0649-0