Abstract
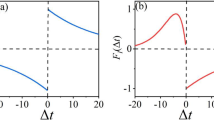
The axons exhibit depolarizing and hyperpolarizing afterpotentials, which result in complicated patterns of recovery cycle and influence the activation of subsequent action potential (AP) with deep brain stimulation (DBS). Our objective is to examine the spike initiation at axonal afterpotentials. We use biophysical models to simulate the afterpotentials and apply two-pulse conditioning-test paradigm to measure the stimulus threshold in recovery cycle. We analyze the phase plane portraits and interactions of ionic currents at spike threshold to determine the spike initiating dynamics associated with the recovery cycle. We show that the afterpotentials alter the net current at voltage threshold of subsequent AP, which results in the changes in spike threshold. The difference between spike threshold and afterpotentials determines the stimulus threshold for evoking subsequent AP, which governs the recovery cycle pattern. Our simulations provide a biophysical basis of the spike initiation at the afterpotentials, which is important for interpreting the activity-dependent modulations of axonal excitability. The predictions should be considered when understanding the frequency-dependent firing patterns in the axon with DBS.











Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this article.
References
Bower, K.L., McIntyre, C.C.: Deep brain stimulation of terminating axons. Brain Stimul. 13(6), 1863–1870 (2020). https://doi.org/10.1016/j.brs.2020.09.001
Lozano, A.M., Lipsman, N., Bergman, H., Brown, P., et al.: Deep brain stimulation: current challenges and future directions. Nat. Rev. Neurol. 15(3), 148–160 (2019). https://doi.org/10.1038/s41582-018-0128-2
Yi, G.S., Wang, J., Wei, X.L., Che, Y.Q.: Energy cost of action potential generation and propagation in thalamocortical relay neurons during deep brain stimulation. IEEE Trans. Biomed. Eng. 66(12), 3457–3471 (2019). https://doi.org/10.1109/TBME.2019.2906114
Kamiya, H.: Excitability tuning of axons by afterdepolarization. Front. Cell. Neurosci. 13, 407 (2019). https://doi.org/10.3389/fncel.2019.00407
Stys, P.K., Waxman, S.G.: Activity-dependent modulation of excitability: implications for axonal physiology and pathophysiology. Muscle Nerve. 17(9), 969–974 (1994). https://doi.org/10.1002/mus.880170902
George, A., Serra, J., Navarro, X., Bostock, H.: Velocity recovery cycles of single C fibres innervating rat skin. J. Physiol. 578(Pt 1), 213–232 (2007). https://doi.org/10.1113/jphysiol.2006.116129
McIntyre, C.C., Grill, W.M.: Extracellular stimulation of central neurons: influence of stimulus waveform and frequency on neuronal output. J. Neurophysiol. 88(4), 1592–1604 (2002). https://doi.org/10.1152/jn.2002.88.4.1592
David, G., Modney, B., Scappaticci, K.A., Barrett, J.N., et al.: Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J. Physiol. 489(Pt 1), 141–157 (1995). https://doi.org/10.1113/jphysiol.1995.sp021037
Barrett, E.F., Barrett, J.N.: Intracellular recording from vertebrate myelinated axons: mechanism of the depolarizing afterpotential. J. Physiol. 323, 117–144 (1982). https://doi.org/10.1113/jphysiol.1982.sp014064
Kiernan, M.C., Mogyoros, I.: Burke, D: Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain 119(Pt 4), 1099–1105 (1996). https://doi.org/10.1093/brain/119.4.1099
Meeks, J.P., Mennerick, S.: Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J. Neurosci. 24(1), 197–206 (2004). https://doi.org/10.1523/jneurosci.4845-03.2004
Kocsis, J.D., Malenka, R.C., Waxman, S.G.: Effects of extracellular potassium concentration on the excitability of the parallel fibres of the rat cerebellum. J. Physiol. 334(1), 225–244 (1983). https://doi.org/10.1113/jphysiol.1983.sp014491
Stephanova, D.I., Daskalova, M., Mladenov, M.: Conducting processes in simulated chronic inflammatory demyelinating polyneuropathy at 20°C-42°C. J. Integr. Neurosci. 14(1), 19–30 (2015). https://doi.org/10.1142/S0219635215500065
Chen, Y.J., Chen, Y.C., Chan, P., Lin, C.I., et al.: Temperature regulates the arrhythmogenic activity of pulmonary vein cardiomyocytes. J. Biomed. Sci. 10(5), 535–543 (2003). https://doi.org/10.1007/BF02256115
Bostock, H., Baker, M.: Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 462(2), 354–358 (1988). https://doi.org/10.1016/0006-8993(88)90564-1
Burke, D., Kiernan, M.C., Bostock, H.: Excitability of human axons. Clin. Neurophysiol. 112(9), 1575–1585 (2001). https://doi.org/10.1016/s1388-2457(01)00595-8
Chiu, S.Y., Ritchie, J.M., Rogart, R.B., Stagg, D.: A quantitative description of membrane currents in rabbit myelinated nerve. J. Physiol. 292, 149–166 (1979). https://doi.org/10.1113/jphysiol.1979.sp012843
Robert, A., Jirounek, P.: Uptake of potassium by nonmyelinating Schwann cells induced by axonal activity. J. Neurophysiol. 72(6), 2570–2579 (1994). https://doi.org/10.1152/jn.1994.72.6.2570
Burke, D., Howells, J., Trevillion, L., McNulty, P.A., et al.: Threshold behaviour of human axons explored using subthreshold perturbations to membrane potential. J. Physiol. 587(2), 491–504 (2009). https://doi.org/10.1113/jphysiol.2008.163170
Tarnaud, T., Joseph, W., Martens, L., Tanghe, E.: Dependence of excitability indices on membrane channel dynamics, myelin impedance, electrode location and stimulus waveforms in myelinated and unmyelinated fibre models. Med. Biol. Eng. Comput. 56(9), 1595–1613 (2018). https://doi.org/10.1007/s11517-018-1799-y
Tai, C.F., de Groat, W.C., Roppolo, J.R.: Simulation analysis of conduction block in unmyelinated axons induced by high-frequency biphasic electrical currents. IEEE Trans. Biomed. Eng. 52(7), 1323–1332 (2005). https://doi.org/10.1109/tbme.2005.847561
Dekker, D.M., Briaire, J.J., Frijns, J.H.: The impact of internodal segmentation in biophysical nerve fiber models. J. Comput. Neurosci. 37(2), 307–315 (2014). https://doi.org/10.1007/s10827-014-0503-y
Yi, G.S., Wang, J., Deng, B., Wei, X.L.: Spike initiating dynamics of the neuron with different adaptation mechanisms to extracellular electric fields. Commun. Nonlinear Sci. Numer. Simul. 22(1–3), 574–586 (2015). https://doi.org/10.1016/j.cnsns.2014.07.020
Yi, G.S., Wang, J., Wei, X.L., Tsang, K.M., et al.: Neuronal spike initiation modulated by extracellular electric fields. PLoS One. 9(5), e97481 (2014). https://doi.org/10.1371/journal.pone.0097481
Tonnelier, A.: Threshold curve for the excitability of bidimensional spiking neurons. Phys. Rev. E 90(2), 022701 (2014). https://doi.org/10.1103/PhysRevE.90.022701
Sekerli, M., Del Negro, C.A., Lee, R.H., Butera, R.J.: Estimating action potential thresholds from neuronal time-series: new metrics and evaluation of methodologies. IEEE Trans. Biomed. Eng. 51(9), 1665–1672 (2004). https://doi.org/10.1109/TBME.2004.827531
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol 117(4), 500–544 (1952). https://doi.org/10.1113/jphysiol.1952.sp004764
Liu, H.L., Roppolo, J.R., de Groat, W.C., Tai, C.F.: The role of slow potassium current in nerve conduction block induced by high-frequency biphasic electrical current. IEEE Trans. Biomed. Eng. 56(1), 137–146 (2009). https://doi.org/10.1109/TBME.2008.2006013
Smit, J.E., Hanekom, T., Hanekom, J.J.: Modelled temperature-dependent excitability behaviour of a generalised human peripheral sensory nerve fibre. Biol. Cybern. 101(2), 115–130 (2009). https://doi.org/10.1007/s00422-009-0324-7
Smit, J.E., Hanekom, T., Wieringen, A., et al.: Threshold predictions of different pulse shapes using a human auditory nerve fibre model containing persistent sodium and slow potassium currents. Hear. Res. 269(1–2), 12–22 (2010). https://doi.org/10.1016/j.heares.2010.08.004
McIntyre, C.C., Richardson, A.G., Grill, W.M.: Modeling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J. Neurophysiol. 87(2), 995–1006 (2002). https://doi.org/10.1152/jn.00353.2001
Tsuboi, R., Mochizuki, T., Ito, H., Kawano, S., et al.: Validation of a lateral flow immunochromatographic assay for tinea unguium diagnosis. J. Dermatol. 48(5), 633–637 (2021). https://doi.org/10.1111/1346-8138.15838
FitzHugh, R.: Mathematical models of threshold phenomena in the nerve membrane. Bull. Math. Biophys. 17, 257–278 (1955). https://doi.org/10.1007/BF02477753
Wang, L.F., Wang, H.T., Yu, L.C., Chen, Y.: Spike-threshold variability originated from separatrix-crossing in neuronal dynamics. Sci. Rep. 6, 31719 (2016). https://doi.org/10.1038/srep31719
Prescott, S.A., Ratté, S., Koninck, Y.D., Sejnowski, T.J.: Nonlinear interaction between shunting and adaptation controls a switch between integration and coincidence detection in pyramidal neurons. J. Neurosci. 26(36), 9084–9097 (2006). https://doi.org/10.1523/JNEUROSCI.1388-06
Coggan, J.S., Prescott, S.A., Bartol, T.M., Sejnowski, T.J.: Imbalance of ionic conductances contributes to diverse symptoms of demyelination. Proc. Natl. Acad. Sci. USA 107(48), 20602–20609 (2010). https://doi.org/10.1073/pnas.1013798107
Platkiewicz, J., Brette, R.: A threshold equation for action potential initiation. PLoS Comput. Biol. 6(7), e1000850 (2010). https://doi.org/10.1371/journal.pcbi.1000850
Ermentrout, B.: Simulating, analyzing, and animating dynamical systems: a guide to XPPAUT for researchers and students. Pennsylvania (2002)
Hines, M.L., Carnevale, N.T.: The NEURON simulation environment. Neural Comput. 9(6), 1179–1209 (1997). https://doi.org/10.1162/neco.1997.9.6.1179
Kiernan, M.C., Mogyoros, I., Burke, D.: Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain 119(Pt 4), 1099–1105 (1996). https://doi.org/10.1093/brain/119.4.1099
Gaines, J.L., Finn, K.E., Slopsema, J.P., Heyboer, L.A., et al.: A model of motor and sensory axon activation in the median nerve using surface electrical stimulation. J. Comput. Neurosci. 45(1), 29–43 (2018). https://doi.org/10.1007/s10827-018-0689-5
Bowe, C.M., Kocsis, J.D., Waxman, S.G.: The association of the supernormal period and the depolarizing afterpotential in myelinated frog and rat sciatic nerve. Neuroscience 21(2), 585–593 (1987). https://doi.org/10.1016/0306-4522(87)90144-8
Hedrich, U.B., Liautard, C., Kirschenbaum, D., Pofahl, M., et al.: Impaired action potential initiation in GABAergic interneurons causes hyperexcitable networks in an epileptic mouse model carrying a human Na(V)1.1 mutation. J. Neurosci. 34(45), 14874–14889 (2014). https://doi.org/10.1523/JNEUROSCI.0721-14.2014
Akgul, G., Wollmuth, L.P.: Synapse-associated protein 97 regulates the membrane properties of fast-spiking parvalbumin interneurons in the visual cortex. J. Neurosci. 33(31), 12739–12750 (2013). https://doi.org/10.1523/JNEUROSCI.0040-13.2013
Helm, J., Akgul, G., Wollmuth, L.P.: Subgroups of parvalbumin-expressing interneurons in layers 2/3 of the visual cortex. J. Neurophysiol. 109(6), 1600–1613 (2013). https://doi.org/10.1152/jn.00782.2012
Guan, D.X., Armstrong, W.E., Foehring, R.C.: Electrophysiological properties of genetically identified subtypes of layer 5 neocortical pyramidal neurons: Ca2+ dependence and differential modulation by norepinephrine. J. Neurophysiol. 113(7), 2014–2032 (2015). https://doi.org/10.1152/jn.00524.2014
Ma, C., Shu, Y.S., Zheng, Z., Chen, Y., et al.: Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J. Neurophysiol. 89(3), 1588–1602 (2003). https://doi.org/10.1152/jn.00855.2002
Kim, C.H., Oh, S.H., Lee, J.H., Chang, S.O., et al.: Lobule-specific membrane excitability of cerebellar Purkinje cells. J. Physiol. 590(2), 273–288 (2012). https://doi.org/10.1113/jphysiol.2011.221846
Jhangiani-Jashanmal, I.T., Yamamoto, R., Gungor, N.Z., Paré, D.: Electroresponsive properties of rat central medial thalamic neurons. J. Neurophysiol. 115(3), 1533–1541 (2016). https://doi.org/10.1152/jn.00982.2015
Farries, M.A., Perkel, D.J.: Electrophysiological properties of avian basal ganglia neurons recorded in vitro. J. Neurophysiol. 84(5), 2502–2513 (2000). https://doi.org/10.1152/jn.2000.84.5.2502
Gonzalez-Burgos, G., Miyamae, T., Pafundo, D.E., Yoshino, H., et al.: Functional maturation of GABA synapses during postnatal development of the monkey dorsolateral prefrontal cortex. Cereb. Cortex 25(11), 4076–4093 (2015). https://doi.org/10.1093/cercor/bhu122
Higgs, M.H., Spain, W.J.: Kv1 channels control spike threshold dynamics and spike timing in cortical pyramidal neurones. J. Physiol. 589(21), 5125–5142 (2011). https://doi.org/10.1113/jphysiol.2011.216721
Lubejko, S.T., Fontaine, B., Soueidan, S.E., MacLeod, K.M.: Spike threshold adaptation diversifies neuronal operating modes in the auditory brain stem. J. Neurophysiol. 122(6), 2576–2590 (2019). https://doi.org/10.1152/jn.00234.2019
Wester, J.C., Contreras, D.: Biophysical mechanism of spike threshold dependence on the rate of rise of the membrane potential by sodium channel inactivation or subthreshold axonal potassium current. J. Comput. Neurosci. 35(1), 1–17 (2013). https://doi.org/10.1007/s10827-012-0436-2
Shvartsman, A., Kotler, O., Stoler, O., Khrapunsky, Y., et al.: Subcellular distribution of persistent sodium conductance in cortical pyramidal neurons. J. Neurosci. 41(29), 6190–6201 (2021). https://doi.org/10.1523/JNEUROSCI.2989-20.2021
Magistretti, J., Castelli, L., Forti, L., D’Angelo, E.: Kinetic and functional analysis of transient, persistent and resurgent sodium currents in rat cerebellar granule cells in situ: an electrophysiological and modelling study. J. Physiol. 573(Pt 1), 83–106 (2006). https://doi.org/10.1113/jphysiol.2006.106682
Xie, R.G., Zheng, D.W., Xing, J.L., Zhang, X.J., et al.: Blockade of persistent sodium currents contributes to the riluzole-induced inhibition of spontaneous activity and oscillations in injured DRG neurons. PLoS One 6(4), e18681 (2011). https://doi.org/10.1371/journal.pone.0018681
Wu, S.N., Lo, Y.C., Chen, B.S., Cheung So, E., et al.: Contribution of blocked potassium current conductance and increased conductance of persistent sodium current to the afterdischarge in myelinated neuron. Muscle Nerve. 46(2), 297–299 (2012). https://doi.org/10.1002/mus.23400
Baker, M., Bostock, H., Grafe, P., Martius, P.: Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J. Physiol. 383, 45–67 (1987). https://doi.org/10.1113/jphysiol.1987.sp016395
Stys, P.K., Ashby, P.: An automated technique for measuring the recovery cycle of human nerves. Muscle Nerve. 13(8), 750–758 (1990). https://doi.org/10.1002/mus.880130814
Hodgkin, A.L., Huxley, A.F., Katz, B.: Measurement of current-voltage relations in the membrane of the giant axon of Loligo. J. Physiol. 116(4), 424–448 (1952). https://doi.org/10.1113/jphysiol.1952.sp004716
Hille, B.: Ion channels of excitable membranes. Massachusetts (2001)
Rinberg, A., Taylor, A.L., Marder, E.: The effects of temperature on the stability of a neuronal oscillator. PLoS Comput. Biol. 9(1), e1002857 (2013). https://doi.org/10.1371/journal.pcbi.1002857
Bostock, H., Cikurel, K., Burke, D.: Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 21(2), 137–158 (1998). https://doi.org/10.1002/(sici)1097-4598(199802)21:2%3c137::aid-mus1%3e3.0.co;2-c
Chiken, S., Nambu, A.: High-frequency pallidal stimulation disrupts information flow through the pallidum by GABAergic inhibition. J. Neurosci. 33(6), 2268–2280 (2013). https://doi.org/10.1523/JNEUROSCI.4144-11.2013
Kuncel, A.M., Cooper, S.E., Wolgamuth, B.R., Grill, W.M.: Amplitude- and frequency-dependent changes in neuronal regularity parallel changes in tremor with thalamic deep brain stimulation. IEEE Trans. Neural. Syst. Rehabil. Eng. 15(2), 190–197 (2017). https://doi.org/10.1109/TNSRE.2007.897004
Yi, G.S., Wang, J.: Frequency-dependent energy demand of dendritic responses to deep brain stimulation in thalamic neurons: a model-based study. IEEE Trans. Neural Netw. Learn. Syst. 32(7), 3056–3068 (2021). https://doi.org/10.1109/TNNLS.2020.3009293
McIntyre, C.C., Grill, W.M., Sherman, D.L., Thakor, N.V.: Cellular effects of deep brain stimulation: modelbased analysis of activation and inhibition. J. Neurophysiol. 91(4), 1457–1469 (2004). https://doi.org/10.1152/jn.00989.2003
Yi, G.S., Grill, W.M.: Frequency-dependent antidromic activation in thalamocortical relay neurons: effects of synaptic inputs. J. Neural. Eng. 15(5), 0556001 (2018). https://doi.org/10.1088/1741-2552/aacbff
Acknowledgements
This work is funded by grants from the National Natural Science Foundation of China (62071324).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yi, G., Zhao, Q. Spike initiating dynamics at axonal afterpotentials: model-based mechanisms of the recovery cycle. Nonlinear Dyn 111, 10487–10504 (2023). https://doi.org/10.1007/s11071-023-08362-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-023-08362-5