Abstract
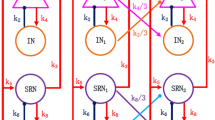
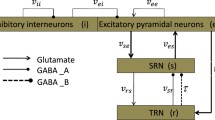
Absence seizures usually occur in dysfunctional neural networks. Thalamic feed-forward inhibition and feed-back inhibition are the two most critical microcircuits, which can be detected in multiple brain regions such as the thalamus and the neocortex. To theoretically explore whether these two inhibition microcircuits have combined effects on absence seizures, we improve corticothalamic mean-field network model through introducing GABAB-mediated inhibitory interneurons in the cerebral cortex (Ctx). On the one hand, we certify that thalamic feed-forward inhibition, i.e., the neurons of the thalamic reticular nucleus (TRN), receive excitatory signals from Ctx and transmit GABAA and GABAB-mediated inhibitory signals to the thalamic relay nucleus (SRN), which plays critical roles in preventing absence seizures. On the other hand, we demonstrate that feed-back inhibition in thalamus, i.e., the neurons of SRN, send excitatory signals to TRN and receive GABAB and GABAA-mediated inhibitory signals from the TRN and also participates in the suppression of absence seizures. Finally, we mainly consider the combined effects of two microcircuits involved in the feed-forward inhibited CTX-TRN pathway and the feed-back inhibited SRN-TRN pathway on absence seizures. Our results show that the two microcircuits play combination roles in eliminating absence seizures. Importantly, feed-back inhibition of thalamus is stronger than feed-forward inhibition in suppressing absence seizures. These results highlight the important significance of two kinds of microcircuit motifs on absence seizures and might provide theoretical guidance for the treatment of epilepsy.









Similar content being viewed by others
Data availability
The MATLAB code and data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Tolaymat, A., Nayak, A., Geyer, J.D., et al.: Diagnosis and management of childhood epilepsy. Curr. Probl. Pediatr. Adolesc. Health Care 45(1), 3–17 (2015)
Wang, Z., Wang, Q.: Stimulation strategies for absence seizures: targeted therapy of the focus in coupled thalamocortical model. Nonlinear Dyn. 96, 1649–1663 (2019)
Yang, C., Liu, Z., Wang, Q., et al.: Epilepsy as a dynamical disorder orchestrated by epileptogenic zone: a review. Nonlinear Dyn. 104, 1901–1916 (2021)
Zuberi, S.M., Symonds, J.D.: Update on diagnosis and management of childhood epilepsies. J. de Pediatria 91(6), 67–77 (2015)
Crunelli, V., Leresche, N.: Childhood absence epilepsy: genes, channels, neurons and networks. Nat. Rev. Neurosci. 3(5), 371–382 (2002)
Depaulis, A., David, O., Charpier, S.: The genetic absence epilepsy rat from Strasbourg as a model to decipher the neuronal and network mechanisms of generalized idiopathic epilepsies. J. Neurosci. Methods 260, 159–174 (2016)
Holt, A.B., Netoff, T.I.: Computational modeling of epilepsy for an experimental neurologist. Exp. Neurol. 244, 75–86 (2013)
Meeren, H., Pijn, J., van Luijtelaar, G., et al.: Cortical focus drives widespread corticothalamic networks during spontaneous absence seizures in rats. J. Neurosci. 22, 1480–1495 (2002)
Avanzini, G., Panzica, F., De Curtis, M.: The role of the thalamus in vigilance and epileptogenic mechanisms. Clin. Neurophysiol. 111(2), S19-26 (2000)
Sysoeva, M., Lüttjohann, A., van Luijtelaar, G., et al.: Dynamics of directional coupling underlying spike-wave discharges. Neuroscience 314, 75–89 (2016)
Lüttjohann, A., van Luijtelaar, G.: The dynamics of cortico-thalamocortical interactions at the transition from pre-ictal to ictal LFPs in absence epilepsy. Neurobiol. Dis. 47(1), 49–60 (2012)
Sysoeva, M., Vinogradova, L., Kuznetsova, G., et al.: Changes in corticocortical and corticohippocampal network during absence seizures in WAG/Rij rats revealed with time varying Granger causality. Epilepsy Behav. 64, 44–50 (2016)
Chen, M., Guo, D., Xia, Y., et al.: Control of absence seizures by the thalamic feed-forward inhibition. Front. Comput. Neurosci. 11, 1–31 (2017)
Fan, D., Zhang, L., Wang, Q.: Transition dynamics and adaptive synchronization of time-delay interconnected corticothalamic systems via nonlinear control. Nonlinear Dyn. 94, 2807–2825 (2018)
Medvedeva, T.M., Sysoeva, M.V., van Luijtelaar, G., et al.: Modeling spike-wave discharges by a complex network of neuronal oscillators. Neural Netw. 98, 271–282 (2018)
Paz, J.T., Davidson, T.J., Frechette, E.S., et al.: Closed-loop optogenetic control of thalamus as a tool for interrupting seizures after cortical injury. Nat. Neurosci. 16(1), 64–70 (2013)
Lüttjohann, A., van Luijtelaar, G.: Thalamic stimulation in absence epilepsy. Epilepsy Res. 106(1–2), 136–145 (2013)
Liu, S., Wang, Q.: Transition dynamics of generalized multiple epileptic seizures associated with thalamic reticular nucleus excitability: a computational study. Commun. Nonlinear Sci. Numer. Simul. 52, 203–213 (2017)
Kros, L., Eelkman Rooda, O.H.J., De Zeeuw, C.I., et al.: Controlling cerebellar output to treat refractory epilepsy. Trends Neurosci. 38(12), 787–799 (2015)
Sorokin, J.M., Davidson, T.J., Frechette, E., et al.: Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron 93(1), 194–210 (2017)
Chen, M., Guo, D., Wang, T., et al.: Bidirectional control of absence seizures by the basal ganglia: a computational evidence. PLoS Comput. Biol. 10(3), 1–17 (2014)
Paz, J.T., Huguenard, J.R.: Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat. Neurosci. 18(3), 351 (2015)
Mccafferty, C., David, F., Venzi, M., et al.: Cortical drive and thalamic feed-forward inhibition control thalamic output synchrony during absence seizures. Nat. Neurosci. 21, 744–756 (2018)
Connors, B.W., Landisman, C.E., Reid, R.C.: Thalamus: organization and function (vol. 1), experimental and clinical aspects (vol. 2). Trends Neurosci. 21(12), 539–540 (1998)
Kohmann, D., Lüttjohann, A., Seidenbecher, T., et al.: Short term depression of gap junctional coupling in reticular thalamic neurons of absence epileptic rats. J. Physiol. 594(19), 5695–5710 (2016)
Wallace, R.H., Marini, C., Petrou, S., et al.: Mutant GABAA receptor 2-subunit in childhood absence epilepsy and febrile seizures. Nat. Genet. 28(1), 49–52 (2001)
Destexhe, A., Contreras, D., Steriade, M.: LTS cells in cerebral cortex and their role in generating spike-and-wave oscillations. Neurocomputing 38–40, 555–563 (2001)
Bortolato, M., Frau, R., Orrù, M., et al.: GABA(B) receptor activation exacerbates spontaneous spike-and-wave discharges in DBA/2J mice. Seizure 19(4), 226–231 (2010)
Marescaux, C., Vergnes, M., Bernasconi, R.: GABAB receptor antagonists: potential new anti-absence drugs. J Neural Transm Suppl. 35, 179–188 (1992)
Robinson, P.A., Rennie, C.J., Rowe, D.L.: Dynamics of large-scale brain activity in normal arousal states and epileptic seizures. Phys. Rev. E 65(4), 1–9 (2002)
Destexhe, A.: Spike-and-wave oscillations based on the properties of GABAB receptors. J. Neurosci. Off. J. Soc. Neurosci. 18(21), 9099–9111 (1998)
Chen, M., Guo, D., Min, L., et al.: Critical roles of the direct GABAergic Pallido- cortical pathway in controlling absence seizures. PLoS Comput. Biol. 11(10), 1–23 (2015)
Rodrigues, S., Barton, D., Szalai, R., et al.: Transitions to spike-wave oscillations and epileptic dynamics in a human cortico-thalamic mean-field model. J. Comput. Neurosci. 27(3), 507–526 (2009)
Marten, F., Rodrigues, S., Benjamin, O., et al.: Onset of polyspike complexes in a mean-field model of human electroencephalography and its application to absence epilepsy. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 367(1891), 1145–1161 (2009)
Paz, J.T., Bryant, A.S., Peng, K., et al.: A new mode of corticothalamic transmission revealed in the Gria4 (-/-) model of absence epilepsy. Nat. Neurosci. 14(9), 1167–1173 (2011)
Shao, Z., Burkhalter, A.: Different Balance of Excitation and Inhibition in Forward and Feedback Circuits of Rat Visual Cortex. J. Neurosci. 16(22), 7353–7365 (1996)
Gluckman, B.J., Nguyen, H., Weinstein, S.L., et al.: Adaptive electric field control of epileptic seizures. J. Neurosci. 21(2), 590–600 (2001)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 12002001) and North China University of Technology Research Fund Program for Key Discipline (No. 110052972027/014).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Duan, L. The combined effects of the thalamic feed-forward inhibition and feed-back inhibition in controlling absence seizures. Nonlinear Dyn 108, 191–205 (2022). https://doi.org/10.1007/s11071-021-07178-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-021-07178-5