Abstract
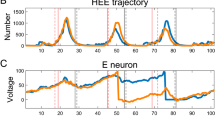
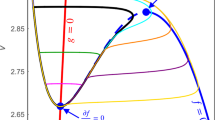
In this paper we present a detailed theoretical analysis of the onset of spike-wave activity in a model of human electroencephalogram (EEG) activity, relating this to clinical recordings from patients with absence seizures. We present a complete explanation of the transition from inter-ictal activity to spike and wave using a combination of bifurcation theory, numerical continuation and techniques for detecting the occurrence of inflection points in systems of delay differential equations (DDEs). We demonstrate that the initial transition to oscillatory behaviour occurs as a result of a Hopf bifurcation, whereas the addition of spikes arises as a result of an inflection point of the vector field. Strikingly these findings are consistent with EEG data recorded from patients with absence seizures and we present a discussion of the clinical significance of these results, suggesting potential new techniques for detection and anticipation of seizures.













Similar content being viewed by others
References
Amari, S. I. (1972). Characteristics of randomly connected threshold-element networks and network systems. Proceedings of the IEEE, 59, 35–47.
Amari, S. I. (1977). Dynamics of pattern formation in lateral-inhibition type neural fields. Biological Cybernetics, 27, 77–87.
Arndt, H. (1984). Numerical solution of retarded initial value problems: Local and global error and stepsize control. Numerical Mathematics, 43, 343–360.
Baker, C. T. H., Paul, C. A. H., & Willé, D. R. (1995). A bibliography on the numerical solution of delay differential equations. Technical Report 269, University of Manchester.
Breakspear, M., Roberts, J. A., Terry, J. R., Rodrigues, S., Mahant, N., & Robinson, P. A. (2006). A unifying explanation of primary generalized seizures through nonlinear brain modeling and bifurcation analysis. Cerebral Cortex, 16, 1296–1313.
Crunelli, V., & Leresche, N. (2002). Childhood absence epilepsy: Genes, channels, neurons and networks. Nature Reviews. Neuroscience, 3, 371–382.
Danober, L., Deransart, C., Depaulis, A., Vergnes, M., & Marescaux, C. (1998). Pathophysiological mechanisms of genetic absence epilepsy in the rat. Progress in Neurobiology, 55, 27–57.
Destexhe, A., & Sejnowski, T. J. (2001). Thalamocortical assemblies. How ion channels, single neurons and large-scale networks organize sleep oscillations. Oxford: Oxford University Press.
Engelborghs, K., Luzyanina, T., & Samaey, G. (2001a). DDE-BIFTOOL v.200 user manual: A M atlab package for bifurcation analysis of delay differential equations. Technical Report tw-330, Department of Computer Science, K. U. Leuven, Leuven, Belgium.
Engelborghs, K., Luzyanina, Hout, K. J., & Roose, D. (2001b). Collocation methods for the computation of periodic solutions of differential equations. SIAM Journal on Scientific Computation, 22(5), 1593–1609.
Freeman, W. J. (1975). Mass action in the nervous system. Examination of the neurophysiological basis of adaptive behavior through the EEG. New York: Academic.
Friston, K. J., Jezzard, P., & Turner, R. (1994). Analysis of functional mri time-series. Human Brain Mapping, 1, 153–171.
Friedrich, R., & Uhl, C. (1996). Spatio-temporal analysis of human electroencephalograms: Petit-mal epilepsy. Physica. D, 98, 171–182.
Geman, S. (1982). Almost sure stable oscillations in a large system of randomly coupled equations. SIAM Journal on Applied Mathematics, 42, 695–703.
Hairer, E., Norsett, S. P., & Wanner, G. (1993). Solving ordinary differential equations I, nonstiff problems. In Springer series computational mathematics (2nd rev. ed.). New York: Springer.
Hairer, E., & Wanner, G. (1996). Solving ordinary differential equations II, stiff and differential algebraic problems. In Springer series computational mathematics (2nd rev. ed.). New York: Springer.
Hernández, J. L., Valdés, P. A., & Vila, P. (1996). EEG spike and wave modelled by a stochastic limit cycle. Neuroreport, 7, 2246–2248.
Hirschberg, P., & Knobloch, E. (1993). Šilnikov-hopf bifurcation. Physica D, 62, 202–216.
Holmes, M. D., Brown, M., & Tucker, D. M. (2004). Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia, 45, 1568–1579.
Jansen, B. H., & Rit, V. G. (1995). Electroencephalogram and visual evoked potential generation in a mathematical model of coupled cortical columns. Biological Cybernetics, 73, 357–366.
Jirsa, V. K., & Haken, H. (1996). Field theory of electromagnetic brain activity. Physical Review Letters, 77, 960–963.
Kim, A. V., & Pimenov, V. G. (1997). Numerical methods for time-delay systems on the basis of i-smooth analysis. Computational Mathematics, 1, 193–196.
Kuznetsov, Y., Kuznetsov, L., & Marsde, J. (1998). Elements of applied bifurcation theory. New York: Springer.
Lopes da Silva, F. H., Hoeks, A., Smits, H., & Zetterberg, L. H. (1974). Model of brain rhythmic activity. The alpha-rhythm of the thalamus. Kybernetik, 15, 27–37.
Lopes da Silva, F. H., & Pijn, J. P. (1999). Epilepsy as a dynamic disease of brain systems. Advances in Neurology, 81, 97–104.
Lopes da Silva, F. H., Blanes, W., Kalitzin, S. N., Parra, J., Suffczynski, P., & Velis, D. N. (2003). Epilepsies as dynamical diseases of brain systems: Basic models of the transition between normal and epileptic activity. Epilepsia, 44, 72–83.
Marescaux, C., Vergnes, M., & Depaulis, A. (1992). Genetic absence epilepsy rat from Strasbourg: A review. Journal of Neural Transmission, 35, 37–69.
Marten, F., Rodrigues, S., Benjamin, O., Richardson, M. P., & Terry, J. R. (2009). Onset of poly-spike complexes in a mean-field model of human EEG and its application to absence epilepsy. Philosophical Transactions of the Royal Society Volume A, 367, 1145–1161.
Milton, J., & Jung, P. (2003). Epilepsy as a dynamic disease. Berlin: Springer.
Nunez, P. L. (1995). Neocortical dynamics and human EEG rhythms. Oxford: Oxford University Press.
Nunez, P. L., & Srinivasan R. (2006). Electric fields of the brain: The neurophysics of EEG (2nd ed.). Oxford: Oxford University Press.
Omurtag, A., Knight, B. W., & Sirovich, L. (2000). On the simulation of large populations of neurons. Journal of Computational Neuroscience, 8, 51–63.
Paul, C. A. H. (1992). Developing a delay differential equation solver. Applied Numerical Mathematics, 9, 403–414.
Robinson, P. A., Rennie, C. J., & Wright, J. J. (1997). Propagation and stability of waves of electrical activity in the cerebral cortex. Physical Review E, 56, 826–840.
Robinson, P. A., Rennie, C. J., Wright, J. J., Bahramali, H., Gordon, E., & Rowe, D. L. (2001). Prediction of electroencephalografic spectra from neurophysiology. Physical Review E, 63, 021903.
Robinson, P. A., Rennie, C. J., & Rowe, D. L. (2002). Dynamics of large-scale brain activity in normal arousal states and epileptic seizures. Physical Review E, 65, 041924.
Rodrigues, S., Terry, J. R., & Breakspear, M. (2006). On the genesis of spike-wave activity in a mean-field model of human thalamic and cortico-thalamic dynamics. Physics Letters A, 355, 352–357.
Rodrigues, S., Barton, D., Szalai, R., Benjamin, O., Richardson, M. P., & Terry, J. R. (2008). Inflection point periodic boundary value problem. http://seis.bris.ac.uk/~enxsr/code.html.
Rozonoér, L. I. (1969). Random logical nets I, II and III. Automation and Remote Control, 5, 773–781 (Translation of Avtomatika i Telemekhanika).
Schiff, N. D., Victor, J. D., Canel, A., & Labar, D. R. (1995). Characteristic nonlinearities of the 3/s ictal electroencephalogram identified by nonlinear autoregressive analysis. Biological Cybernetics, 72, 519–526.
Sieber, J., & Krauskopf, B. (2008). Control-based bifurcation analysis for experiments. Nonlinear Dynamics, 51(3), 365–377.
Steriade M., Amzica, F., Neckelmann, D., & Timofeev, I. (1998). Spike-wave complexes and fast components of cortically generated seizures. ii. extra- and intracellular patterns. Journal of Neurophysiology, 80, 1456–1479.
Steriade, M., & Contreras, D. (1998). Spike-wave complexes and fast components of cortically generated seizures. I. role of neocortex and thalamus. Journal of Neurophysiology, 80, 1439–1455.
Suffczynski, P. (2000). Neural dynamics underlying brain thalamic oscillations investigated with computational models. Ph.D. Diss., Institute of experimental physics, Department of Physics, Warsaw University.
Suffczynski, P., Kalitzin, S., & da Silva, F. H. L. (2004). Dynamics of non-convulsive epileptic phenomena modeled by a bistable neuronal network. Journal of Neuroscience, 126, 467–484.
Szalai, R., Stépán, G., & Hogan, S. J. (2006). Continuation of bifurcations in periodic delay-differential equations using characteristic matrices. SIAM Journal on Scientific Computing, 4, 1301–1317.
van Luijtelaar, E. L., & Coenen, A. M. L. (1986). Two types of electrocortical paroxysms in an inbred strain of rats. Neuroscience Letters, 70, 393–397.
van Luijtelaar, E. L. J. M., & Coenen, A. M. L. (2003). Genetic animal models for absence epilepsy: A review of the WAG/Rij strain of rats. Behavior Genetics, 33, 635–655.
Wang, X. J., Golomb, D., & Rinzel, J. (1995). Emergent spindle oscillations and intermittent bursts firing in a thalamic model: Specific neuronal parameters. Proceedings of the National Academy of Sciences of the United States of America, 92, 5577–5581.
Wendling, F., Bartolomei, F., Bellanger, J. J., F. B., & Chauvel, P. (2002). Epileptic fast activity can be explained by a model of impaired gabaergic dendritic inhibition. European Journal of Neuroscience, 15, 1499–1508.
Wilson, H. R., & Cowan, J. D. (1972). Excitatory and inhibitory interactions in localized populations of model neurons. Biophysical Journal, 12, 1–24.
Wright, J. J., & Liley, D. J. (1996). Dynamics of the brain at global and microscopic scales: Neural networks and the EEG. Behavioral Brain Science, 19, 285–320.
Acknowledgements
SR would like to thank Mathieu Desroches and Frank Marten for some very helpful discussions. SR, MPR and JRT acknowledge financial support from the Leverhulme Trust Theoretical Neuroscience Network and the EPSRC via grant EP/D068436/01. We should like to thank the referees for their useful suggestions which have strengthened the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Action Editor: Steven J. Schiff
Appendix A
Appendix A
1.1 A.1 Mean-field cortico-thalamic model
The global(spatially invariant) mean-field delayed cortico-thalamic model is expressed as follows:
The principal state variables are ϕ e (mean excitatory cortical field), V s (averaged membrane voltage of the thalamic specific neurons) and V r (membrane voltage of thalamic relay neurons). External sensory inputs or noise is projected onto the specific relay neurons.
1.2 A.2 Initial conditions employed in the model
These initial conditions are defined through linear stability analysis (Robinson et al. 2002), which allows to place the system (11) in some unstable region of attraction where seizure can occur. Denoting the cortico-thalamic model (11) by Eq. (8), then the initial condition φ(t) is
where < random > is a continuous random variable sampled from a uniform probability density function and the inverse sigmoidal is defined in the following way
and the pseudo-code for the Vs(.) function follows the Newton’s iteration scheme defined as
-
function Vs(double a)[
integer i;
integer namx=10;
double root=0;
for(i=0; i< namx; i++)[
x=nu_re*varsigma(a) + nu_rs*varsigma(root);
ff=-root+*nu_sn*phi_n + nu_se*varsigma(a) + nu_sr*varsigma(x);
fp=-1+nu_sr*nu_rs*varsigma^{’}(x)*varsigma^{’}(root);
root=root-ff/fp;
]
return (root);
]
Note that we run our simulations a constant input signal. That is the variance of the noise that projects to the specific relay cells is zeros, ϕ n = 1. ς ′ is the derivative of ς with respect to voltage, which is defined as follows
1.3 A.3 Parameters values employed in the model
The parameters values used to simulate absence seizures are given in the following table:
1.4 A.4 Periodic boundary value problem
Mathematically, the periodic boundary value problem with the inflection point condition is defined as follows
Here we have rescaled the vector field F(.) of the model (11), so that its time scale, corresponds to that of period T, giving the term x(t − τ/T). ν is the set of model’s parameters and the parameter, T, is the period of oscillation as determined by the boundary value problem. The periodicity condition is given by x(0) = x(1), that is periodic oscillations are rescaled to the time interval [0, 1]. The final equation defines the conditions for an inflection to occur on the first component of the vector field, f 1. To numerically solve (15), a descretisation scheme is required for which we use the collocation method (Engelborghs et al. 2001b). The exact implementation in DDE-BIFTOOL of the periodic boundary condition can be obtained at the following website (Rodrigues et al. 2008).
Rights and permissions
About this article
Cite this article
Rodrigues, S., Barton, D., Szalai, R. et al. Transitions to spike-wave oscillations and epileptic dynamics in a human cortico-thalamic mean-field model. J Comput Neurosci 27, 507–526 (2009). https://doi.org/10.1007/s10827-009-0166-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10827-009-0166-2