Abstract
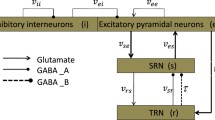
Motivated by the recent experimental findings that thalamic reticular nucleus (TRN) may be a pacemaker of absence seizures, we explore whether changes in the level of TRN activation can induce absence seizures by using a coupled thalamocortical model. We first simulate different firing states by considering the interaction of pathway between cortical excitatory pyramidal neuronal population (PY)–TRN and specific relay nucleus (SRN)–TRN. By simultaneously increasing the coupling strength of each of these pathways, we can reproduce the absence seizures, which indicates that epileptic seizures may be caused by activating the TRN. We further infer that the TRN may be an epileptogenic focus. Following this, different stimulation strategies, including deep brain stimulation, 1:0 coordinated reset stimulation (CRS) and 3:2 CRS, are applied in TRN. By qualitatively analyzing the efficacy of three different stimulation methods, we find that 3:2 CRS is a more effective and safe method to control absence seizures in the first compartment, for which we then further explore the impact of 3:2 CRS in the second compartment. The results show that the additional stimulation in the second compartment also can lead to a considerable decrease in the spike-and-wave discharges (SWD) oscillation region. Therefore, we conclude that TRN-3:2 CRS is an optimal electrical stimulation method for our modeling and simulation studies. Furthermore, we hope that these numerical simulation results can provide some references for the treatment of real epilepsy patients in the future.









Similar content being viewed by others
References
Barad, Z., Grattan, D.R., Leitch, B.: NMDA receptor expression in the thalamus of the stargazer model of absence epilepsy. Sci. Rep. 7, 42926 (2017)
Liu, S., Wang, Q.: Transition dynamics of generalized multiple epileptic seizures associated with thalamic reticular nucleus excitability: a computational study. Commun. Nonlinear Sci. Numer. Simul. 52, 203–213 (2017)
Guerrini, R., Melani, F., Brancati, C., et al.: Dysgraphia as a mild expression of dystonia in children with absence epilepsy. PLoS ONE 10(7), e0130883 (2015)
Polack, P.O., Guillemain, I., Hu, E., et al.: Deep layer somatosensory cortical neurons initiate spike-and-wave discharges in a genetic model of absence seizures. J. Neurosci. 27(24), 6590–6599 (2007)
Steriade, M., Contreras, D.: Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J. Neurophysiol. 80(3), 1439–1455 (1998)
Liu, Z., Vergnes, M., Depaulis, A., et al.: Involvement of intrathalamic \(\text{ GABA }_{{\rm B}}\) neurotransmission in the control of absence seizures in the rat. Neuroscience 48(1), 87–93 (1992)
Liu, Z., Vergnes, M., Depaulis, A., et al.: Evidence for a critical role of GABAergic transmission within the thalamus in the genesis and control of absence seizures in the rat. Brain Res. 545(1–2), 1–7 (1991)
Markram, H., Toledo-Rodriguez, M., Wang, Y., et al.: Interneurons of the neocortical inhibitory system. Nat. Rev. Neurosci. 5(10), 793 (2004)
Adotevi, N.K., Leitch, B.: Alterations in AMPA receptor subunit expression in cortical inhibitory interneurons in the epileptic stargazer mutant mouse. Neuroscience 339, 124–138 (2016)
Mineff, E.M., Weinberg, R.J.: Differential synaptic distribution of AMPA receptor subunits in the ventral posterior and reticular thalamic nuclei of the rat. Neuroscience 101(4), 969–982 (2000)
Trevelyan, A.J., Sussillo, D., Watson, B.O., et al.: Modular propagation of epileptiform activity: evidence for an inhibitory veto in neocortex. J. Neurosci. 26(48), 12447–12455 (2006)
Cammarota, M., Losi, G., Chiavegato, A., et al.: Fast spiking interneuron control of seizure propagation in a cortical slice model of focal epilepsy. J. Physiol. 591(4), 807–822 (2013)
Schevon, C.A., Weiss, S.A., McKhann Jr., G., et al.: Evidence of an inhibitory restraint of seizure activity in humans. Nat. Commun. 3, 1060 (2012)
Shiri, Z., Manseau, F., Lévesque, M., et al.: Interneuron activity leads to initiation of lowvoltage fastonset seizures. Ann. Neurol. 77(3), 541–546 (2015)
Yekhlef, L., Breschi, G.L., Lagostena, L., et al.: Selective activation of parvalbumin-or somatostatin-expressing interneurons triggers epileptic seizurelike activity in mouse medial entorhinal cortex. J. Neurophysiol. 113(5), 1616–1630 (2014)
Sessolo, M., Marcon, I., Bovetti, S., et al.: Parvalbumin-positive inhibitory interneurons oppose propagation but favor generation of focal epileptiform activity. J. Neurosci. 35(26), 9544–9557 (2015)
Kwan, P., Brodie, M.J.: Early identification of refractory epilepsy. N. Engl. J. Med. 342(5), 314–319 (2000)
Engel, J., Wiebe, S., French, J., et al.: Practice parameter: temporal lobe and localized neocortical resections for epilepsy. Epilepsia 44(6), 741–751 (2003)
Kwan, P., Arzimanoglou, A., Berg, A.T., et al.: Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia 51(6), 1069–1077 (2010)
Pantoja-Jiménez, C.R., Magdaleno-Madrigal, V.M., Almazán-Alvarado, S., et al.: Anti-epileptogenic effect of high-frequency stimulation in the thalamic reticular nucleus on PTZ-induced seizures. Brain Stimul. 7(4), 587–594 (2014)
Lehtimäki, K., Långsjö, J.W., Ollikainen, J., et al.: Successful management of super-refractory status epilepticus with thalamic deep brain stimulation. Ann. Neurol. 81(1), 142–146 (2017)
Nanobashvili, Z., Chachua, T., Nanobashvili, A., et al.: Suppression of limbic motor seizures by electrical stimulation in thalamic reticular nucleus. Exp. Neurol. 181(2), 224–230 (2003)
Wang, Z., Wang, Q.: Eliminating absence seizures through the deep brain stimulation to thalamus reticular nucleus. Front. Comput. Neurosci. 11, 22 (2017)
Zeitler, M., Tass, P.A.: Anti-kindling induced by two-stage coordinated reset stimulation with weak onset intensity. Front. Comput. Neurosci. 10(154), (2016)
Zeitler, M., Tass, P.A.: Augmented brain function by coordinated reset stimulation with slowly varying sequences. Front. Syst. Neurosci. 9, 49 (2015)
Fan, D., Wang, Q.: Improving desynchronization of parkinsonian neuronal network via triplet-structure coordinated reset stimulation. J. Theor. Biol. 370, 157–170 (2015)
Wang, Z.H., Wang, Q.Y.: Effect of the coordinated reset stimulations on controlling absence seizure. Sci. China Technol. Sci. 60(7), 985–994 (2017)
Dow, R.S., Fernández-Guardiola, A., Manni, E.: The influence of the cerebellum on experimental epilepsy. Electroencephalogr. Clin. Neurophysiol. 14(3), 383–398 (1962)
Mirski, M.A., Rossell, L.A., Terry, J.B., et al.: Anticonvulsant effect of anterior thalamic high frequency electrical stimulation in the rat. Epilepsy Res. 28(2), 89–100 (1997)
Berdiev, R.K., Luijtelaar, G.V.: Cholinergic stimulation of the nucleus basalis of Meynert and reticular thalamic nucleus affects spike-and-wave discharges in WAG/Rij rats. Neurosci. Lett. 463(3), 249–253 (2009)
Pantojajiménez, C.R., Magdalenomadrigal, V.M., Almazánalvarado, S., et al.: Anti-epileptogenic effect of high-frequency stimulation in the thalamic reticular nucleus on PTZ-induced seizures. Brain Stimul. 7(4), 587–594 (2014)
Fisher, R., Salanova, V., Witt, T., et al.: Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia 51(5), 899–908 (2010)
Taylor, P.N., Baier, G.: A spatially extended model for macroscopic spike-wave discharges. J. Comput. Neurosci. 31(3), 679–684 (2011)
Goodfellow, M., Schindler, K., Baier, G.: Intermittent spike-wave dynamics in a heterogeneous, spatially extended neural mass model. Neuroimage 55(3), 920–932 (2011)
Taylor, P.N., Baier, G., Cash, S.S., et al.: A model of stimulusinduced epileptic spike-wave discharges. In: 2013 IEEE Symposium on Computational Intelligence, Cognitive Algorithms, Mind, and Brain (CCMB), pp. 53–59. IEEE (2013)
Yan, B., Li, P.: An integrative view of mechanisms underlying generalized spike-and-wave epileptic seizures and its implication on optimal therapeutic treatments. PLoS ONE. 6(7), e22440 (2011)
Taylor, P.N., Wang, Y., Goodfellow, M., et al.: A computational study of stimulus driven epileptic seizure abatement. PLoS ONE 9(12), e114316 (2014)
Evangelista, E., Bénar, C., Bonini, F., et al.: Does the thalamo-cortical synchrony play a role in seizure termination? Front. Neurol. 6, 192 (2015)
Moeller, F., Muthuraman, M., Stephani, U., et al.: Representation and propagation of epileptic activity in absences and generalized photoparoxysmal responses. Human Brain Map. 34(8), 1896–1909 (2013)
Neal, T.P., Wang, Y., Marc, G., et al.: A computational study of stimulus driven epileptic seizure abatement. PLoS ONE 9(12), e114316 (2014)
Taylor, P.N., Thomas, J., Sinha, N., et al.: Optimal control based seizure abatement using patient derived connectivity. Front. Neurosci. 9(9), 202 (2015)
Ellis, T.L., Stevens, A.: Deep brain stimulation for medically refractory epilepsy. Neurosurg. Focus 25(3), E11 (2008)
McConnell, G.C., So, R.Q., Hilliard, J.D., et al.: Effective deep brain stimulation suppresses low-frequency network oscillations in the basal ganglia by regularizing neural firing patterns. J. Neurosci. 32(45), 15657–15668 (2012)
Benazzouz, A., Piallat, B., Pollak, P., et al.: Responses of substantia nigra pars reticulata and globus pallidus complex to high frequency stimulation of the subthalamic nucleus in rats: electrophysiological data. Neurosci. Lett. 189(2), 77–80 (1995)
Feddersen, B., Vercueil, L., Noachtar, S., et al.: Controlling seizures is not controlling epilepsy: a parametric study of deep brain stimulation for epilepsy. Neurobiol. Dis. 27(3), 292–300 (2007)
Vercueil, L., Benazzouz, A., Deransart, C., et al.: High-frequency stimulation of the sub-thalamic nucleus suppresses absence seizures in the rat: comparison with neurotoxic lesions. Epilepsy Res. 31(1), 39–46 (1998)
Fan, D., Wang, Z., Wang, Q.: Optimal control of directional deep brain stimulation in the parkinsonian neuronal network. Commun. Nonlinear Sci. Numer. Simul. 36, 219–237 (2016)
Chen, M., Guo, D., Wang, T., et al.: Bidirectional control of absence seizures by the basal ganglia: a computational evidence. PLoS Comput. Biol. 10(3), e1003495 (2014)
Chen, M., Guo, D., Li, M., et al.: Critical roles of the direct GABAergic pallido-cortical pathway in controlling absence seizures. PLoS Comput. Biol. 11(10), e1004539 (2015)
Lewis, L.D., Voigts, J., Flores, F.J., Schmitt, L.I., Wilson, M.A., Halassa, M.M., Brown, E.N.: Thalamic reticular nucleus induces fast and local modulation of arousal state. Elife 13(4), e08760 (2015)
John, Y.J., Zikopoulos, B., Bullock, D., Barbas, H.: The emotional gatekeeper: a computational model of attentional selection and suppression through the pathway from the amygdala to the inhibitory thalamic reticular nucleus. PLoS Comput. Biol. 12(2), e1004722 (2016)
Perry, M.S., Duchowny, M.: Surgical versus medical treatment for refractory epilepsy: outcomes beyond seizure control. Epilepsia 54(12), 2060–2070 (2013)
Irimia, A., Van Horn, J.D.: Epileptogenic focus localization in treatment-resistant post-traumatic epilepsy. J. Clin. Neurosci. 22(4), 627–631 (2015)
Fan, D., Wang, Q.: Improved control effect of absence seizures by autaptic connections to the subthalamic nucleus. Phys. Rev. E 98(5), 052414 (2018)
Liang, S., Wang, Z.H.: Controlling a neuron by stimulating a coupled neuron. Appl. Math. Mech. Engl. Ed. 40(1), 13–24 (2019)
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 11772019).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human and animals rights
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, Z., Wang, Q. Stimulation strategies for absence seizures: targeted therapy of the focus in coupled thalamocortical model. Nonlinear Dyn 96, 1649–1663 (2019). https://doi.org/10.1007/s11071-019-04876-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-019-04876-z