Abstract
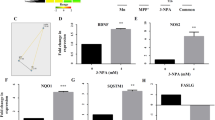
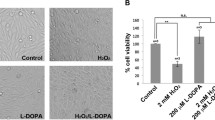
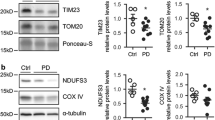
Mitochondrial dysfunction and oxidative stress are critical to neurodegeneration in Parkinson’s disease (PD). Mitochondrial dysfunction in PD entails inhibition of the mitochondrial complex I (CI) in the dopaminergic neurons of substantia nigra. The events contributing to CI inhibition and downstream pathways are not completely elucidated. We conducted proteomic analysis in a dopaminergic neuronal cell line exposed individually to neurotoxic CI inhibitors: rotenone (Rot), paraquat (Pq) and 1-methyl-4-phenylpyridinium (MPP+). Mass spectrometry (MS) revealed the involvement of biological processes including cell death pathways, structural changes and metabolic processes among others, most of which were common across all models. The proteomic changes induced by Pq were significantly higher than those induced by Rot and MPP+. Altered metabolic processes included downregulated mitochondrial proteins such as CI subunits. MS of CI isolated from the models revealed oxidative post-translational modifications with Tryptophan (Trp) oxidation as the predominant modification. Further, 62 peptides in 22 subunits of CI revealed Trp oxidation with 16 subunits common across toxins. NDUFV1 subunit had the greatest number of oxidized Trp and Rot model displayed the highest number of Trp oxidation events compared to the other models. Molecular dynamics simulation (MDS) of NDUFV1 revealed that oxidized Trp 433 altered the local conformation thereby changing the distance between the Fe-S clusters, Fe-S 301(N1a) to Fe-S 502 (N3) and Fe-S 802 (N4) to Fe-S 801 (N5), potentially affecting the efficiency of electron transfer. The events triggered by the neurotoxins represent CI damage, mitochondrial dysfunction and neurodegeneration in PD.












Similar content being viewed by others
Data Availability
The proteomics (MS) data from this study have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository [81] with the dataset identifier PXD037322.
References
Perry TL, Godin DV, Hansen S (1982) Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett 33:305–310. https://doi.org/10.1016/0304-3940(82)90390-1
Schapira AH, Mann VM, Cooper JM et al (1990) Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem 55:2142–2145. https://doi.org/10.1111/j.1471-4159.1990.tb05809.x
Swerdlow RH, Parks JK, Miller SW et al (1996) Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol 40:663–671. https://doi.org/10.1002/ana.410400417
Lazarou M, Thorburn DR, Ryan MT, McKenzie M (2009) Assembly of mitochondrial complex I and defects in disease. Biochim Biophys Acta 1793:78–88. https://doi.org/10.1016/j.bbamcr.2008.04.015
Swalwell H, Kirby DM, Blakely EL et al (2011) Respiratory chain complex I deficiency caused by mitochondrial DNA mutations. Eur J Hum Genet 19:769–775. https://doi.org/10.1038/ejhg.2011.18
Srinivas Bharath MM (2017) Post-translational oxidative modifications of mitochondrial complex I (NADH: ubiquinone oxidoreductase): implications for pathogenesis and therapeutics in human diseases. J Alzheimers Dis 60:S69–S86. https://doi.org/10.3233/JAD-170117
Sunitha B, Gayathri N, Kumar M et al (2016) Muscle biopsies from human muscle diseases with myopathic pathology reveal common alterations in mitochondrial function. J Neurochem 138:174–191. https://doi.org/10.1111/jnc.13626
Nandipati S, Litvan I (2016) Environmental exposures and Parkinson’s disease. Int J Environ Res Public Health 13:881. https://doi.org/10.3390/ijerph13090881
Prasad KN, Carvalho E, Kentroti S et al (1994) Establishment and characterization of immortalized clonal cell lines from fetal rat mesencephalic tissue. In Vitro Cell Dev Biol Anim 30A:596–603. https://doi.org/10.1007/BF02631258
Mythri RB, Jagatha B, Pradhan N et al (2007) Mitochondrial complex I inhibition in Parkinson’s disease: how can curcumin protect mitochondria? Antioxid Redox Signal 9:399–408. https://doi.org/10.1089/ars.2006.1479
Vali S, Mythri RB, Jagatha B et al (2007) Integrating glutathione metabolism and mitochondrial dysfunction with implications for Parkinson’s disease: a dynamic model. Neuroscience 149:917–930. https://doi.org/10.1016/j.neuroscience.2007.08.028
Chan FK-M, Moriwaki K, De Rosa MJ (2013) Detection of necrosis by release of lactate dehydrogenase activity. Methods Mol Biol 979:65–70. https://doi.org/10.1007/978-1-62703-290-2_7
Harish G, Venkateshappa C, Mythri RB et al (2010) Bioconjugates of curcumin display improved protection against glutathione depletion mediated oxidative stress in a dopaminergic neuronal cell line: implications for Parkinson’s disease. Bioorg Med Chem 18:2631–2638. https://doi.org/10.1016/j.bmc.2010.02.029
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1006/abio.1976.9999
Rappsilber J, Mann M, Ishihama Y (2007) Protocol for micro-purification, enrichment, pre-fractionation and storage of peptides for proteomics using StageTips. Nat Protoc 2:1896–1906. https://doi.org/10.1038/nprot.2007.261
Trounce IA, Kim YL, Jun AS, Wallace DC (1996) Assessment of mitochondrial oxidative phosphorylation in patient muscle biopsies, lymphoblasts, and transmitochondrial cell lines. Methods Enzymol 264:484–509. https://doi.org/10.1016/s0076-6879(96)64044-0
Butterfield DA, Stadtman ER (1997) Chapter 7 protein oxidation processes in aging brain. In: Timiras PS, Bittar EE (eds) Advances in cell aging and gerontology. Elsevier, pp 161–191
Ryan K, Backos DS, Reigan P, Patel M (2012) Post-translational oxidative modification and inactivation of mitochondrial complex I in epileptogenesis. J Neurosci 32:11250–11258. https://doi.org/10.1523/JNEUROSCI.0907-12.2012
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Vanommeslaeghe K, Hatcher E, Acharya C et al (2010) CHARMM general force field: a force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J Comput Chem 31:671–690. https://doi.org/10.1002/jcc.21367
Zeng X-S, Geng W-S, Jia J-J (2018) Neurotoxin-induced animal models of Parkinson disease: pathogenic mechanism and assessment. ASN Neuro 10:1759091418777438. https://doi.org/10.1177/1759091418777438
Mythri RB, Raghunath NR, Narwade SC et al (2017) Manganese- and 1-methyl-4-phenylpyridinium-induced neurotoxicity display differences in morphological, electrophysiological and genome-wide alterations: implications for idiopathic Parkinson’s disease. J Neurochem 143:334–358. https://doi.org/10.1111/jnc.14147
Mohankumar T, Chandramohan V, Lalithamba HS et al (2020) Design and molecular dynamic investigations of 7,8-dihydroxyflavone derivatives as potential neuroprotective agents against alpha-synuclein. Sci Rep 10:599. https://doi.org/10.1038/s41598-020-57417-9
Zurita Rendón O, Silva Neiva L, Sasarman F, Shoubridge EA (2014) The arginine methyltransferase NDUFAF7 is essential for complex I assembly and early vertebrate embryogenesis. Hum Mol Genet 23:5159–5170. https://doi.org/10.1093/hmg/ddu239
Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. Transl Neurodegener 6:28. https://doi.org/10.1186/s40035-017-0099-z
van Dijk KD, Berendse HW, Drukarch B et al (2012) The proteome of the locus ceruleus in Parkinson’s disease: relevance to pathogenesis. Brain Pathol 22:485–498. https://doi.org/10.1111/j.1750-3639.2011.00540.x
Basso M, Giraudo S, Corpillo D et al (2004) Proteome analysis of human substantia nigra in Parkinson’s disease. Proteomics 4:3943–3952. https://doi.org/10.1002/pmic.200400848
Tribl F, Gerlach M, Marcus K et al (2005) “Subcellular proteomics” of neuromelanin granules isolated from the human brain. Mol Cell Proteom 4:945–957. https://doi.org/10.1074/mcp.M400117-MCP200
Palacino JJ, Sagi D, Goldberg MS et al (2004) Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem 279:18614–18622. https://doi.org/10.1074/jbc.M401135200
Periquet M, Corti O, Jacquier S, Brice A (2005) Proteomic analysis of parkin knockout mice: alterations in energy metabolism, protein handling and synaptic function. J Neurochem 95:1259–1276. https://doi.org/10.1111/j.1471-4159.2005.03442.x
Poon HF, Frasier M, Shreve N et al (2005) Mitochondrial associated metabolic proteins are selectively oxidized in A30P alpha-synuclein transgenic mice–a model of familial Parkinson’s disease. Neurobiol Dis 18:492–498. https://doi.org/10.1016/j.nbd.2004.12.009
Heeman B, Van den Haute C, Aelvoet S-A et al (2011) Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci 124:1115–1125. https://doi.org/10.1242/jcs.078303
Yao Z, Gandhi S, Burchell VS et al (2011) Cell metabolism affects selective vulnerability in PINK1-associated Parkinson’s disease. J Cell Sci 124:4194–4202. https://doi.org/10.1242/jcs.088260
Triplett JC, Zhang Z, Sultana R et al (2015) Quantitative expression proteomics and phosphoproteomics profile of brain from PINK1 knockout mice: insights into mechanisms of familial Parkinson’s disease. J Neurochem 133:750–765. https://doi.org/10.1111/jnc.13039
Karthikkeyan G, Najar MA, Pervaje R et al (2020) Identification of molecular network associated with neuroprotective effects of Yashtimadhu (Glycyrrhiza glabra L.) by quantitative proteomics of rotenone-induced Parkinson’s disease model. ACS Omega 5:26611–26625. https://doi.org/10.1021/acsomega.0c03420
Gielisch I, Meierhofer D (2015) Metabolome and proteome profiling of complex I deficiency induced by rotenone. J Proteome Res 14:224–235. https://doi.org/10.1021/pr500894v
Ranganayaki S, Jamshidi N, Aiyaz M et al (2021) Inhibition of mitochondrial complex II in neuronal cells triggers unique pathways culminating in autophagy with implications for neurodegeneration. Sci Rep 11:1483. https://doi.org/10.1038/s41598-020-79339-2
Beal MF (2003) Mitochondria, oxidative damage, and inflammation in Parkinson’s disease. Ann N Y Acad Sci 991:120–131. https://doi.org/10.1111/j.1749-6632.2003.tb07470.x
De Lazzari F, Bubacco L, Whitworth AJ, Bisaglia M (2018) Superoxide radical dismutation as new therapeutic strategy in Parkinson’s disease. Aging Dis 9:716–728. https://doi.org/10.14336/AD.2017.1018
Flynn JM, Melov S (2013) SOD2 in mitochondrial dysfunction and neurodegeneration. Free Radic Biol Med 62:4–12. https://doi.org/10.1016/j.freeradbiomed.2013.05.027
Sheng Z-H (2014) Mitochondrial trafficking and anchoring in neurons: new insight and implications. J Cell Biol 204:1087–1098. https://doi.org/10.1083/jcb.201312123
Lippolis R, Siciliano RA, Pacelli C et al (2015) Altered protein expression pattern in skin fibroblasts from parkin-mutant early-onset Parkinson’s disease patients. Biochim Biophys Acta 1852:1960–1970. https://doi.org/10.1016/j.bbadis.2015.06.015
Suzuki S, Numakawa T, Shimazu K et al (2004) BDNF-induced recruitment of TrkB receptor into neuronal lipid rafts: roles in synaptic modulation. J Cell Biol 167:1205–1215. https://doi.org/10.1083/jcb.200404106
Bhatnagar A, Sheffler DJ, Kroeze WK et al (2004) Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Galphaq-coupled protein receptors. J Biol Chem 279:34614–34623. https://doi.org/10.1074/jbc.M404673200
Francesconi A, Kumari R, Zukin RS (2009) Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci 29:3590–3602. https://doi.org/10.1523/JNEUROSCI.5824-08.2009
Head BP, Peart JN, Panneerselvam M et al (2010) Loss of caveolin-1 accelerates neurodegeneration and aging. PLoS ONE 5:e15697. https://doi.org/10.1371/journal.pone.0015697
Wang S, Wang N, Zheng Y et al (2017) Caveolin-1: an oxidative stress-related target for cancer prevention. Oxid Med Cell Longev 2017:7454031. https://doi.org/10.1155/2017/7454031
Surmeier DJ, Guzman JN, Sanchez-Padilla J, Schumacker PT (2011) The role of calcium and mitochondrial oxidant stress in the loss of substantia nigra pars compacta dopaminergic neurons in Parkinson’s disease. Neuroscience 198:221–231. https://doi.org/10.1016/j.neuroscience.2011.08.045
Hurley MJ, Brandon B, Gentleman SM, Dexter DT (2013) Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 136:2077–2097. https://doi.org/10.1093/brain/awt134
Donato R (1999) Functional roles of S100 proteins, calcium-binding proteins of the EF-hand type. Biochim Biophys Acta 1450:191–231. https://doi.org/10.1016/s0167-4889(99)00058-0
Pankratova S, Klingelhofer J, Dmytriyeva O et al (2018) The S100A4 protein signals through the ErbB4 receptor to promote neuronal survival. Theranostics 8:3977–3990. https://doi.org/10.7150/thno.22274
An N, Bassil K, Al Jowf GI et al (2021) Dual-specificity phosphatases in mental and neurological disorders. Prog Neurobiol 198:101906. https://doi.org/10.1016/j.pneurobio.2020.101906
Khoubai FZ, Grosset CF (2021) DUSP9, a dual-specificity phosphatase with a key role in cell biology and human diseases. Int J Mol Sci 22:11538. https://doi.org/10.3390/ijms222111538
Luo G-R, Chen S, Le W-D (2006) Are heat shock proteins therapeutic target for Parkinson’s disease? Int J Biol Sci 3:20–26. https://doi.org/10.7150/ijbs.3.20
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316. https://doi.org/10.1074/jbc.272.33.20313
Butterfield DA, Palmieri EM, Castegna A (2016) Clinical implications from proteomic studies in neurodegenerative diseases: lessons from mitochondrial proteins. Expert Rev Proteom 13:259–274. https://doi.org/10.1586/14789450.2016.1149470
Danielson SR, Andersen JK (2008) Oxidative and nitrative protein modifications in Parkinson’s disease. Free Radic Biol Med 44:1787–1794. https://doi.org/10.1016/j.freeradbiomed.2008.03.005
Danielson SR, Held JM, Oo M et al (2011) Quantitative mapping of reversible mitochondrial Complex I cysteine oxidation in a Parkinson disease mouse model. J Biol Chem 286:7601–7608. https://doi.org/10.1074/jbc.M110.190108
Butterfield DA, Boyd-Kimball D (2019) Redox proteomics and amyloid β-peptide: insights into Alzheimer disease. J Neurochem 151:459–487. https://doi.org/10.1111/jnc.14589
Newman SF, Sultana R, Perluigi M et al (2007) An increase in S-glutathionylated proteins in the Alzheimer’s disease inferior parietal lobule, a proteomics approach. J Neurosci Res 85:1506–1514. https://doi.org/10.1002/jnr.21275
Hurd TR, Requejo R, Filipovska A et al (2008) Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J Biol Chem 283:24801–24815. https://doi.org/10.1074/jbc.M803432200
Murray J, Taylor SW, Zhang B et al (2003) Oxidative damage to mitochondrial complex I due to peroxynitrite: identification of reactive tyrosines by mass spectrometry. J Biol Chem 278:37223–37230. https://doi.org/10.1074/jbc.M305694200
Taylor Oxidative post-translational modification of tryptophan residues in cardiac mitochondrial proteins - PubMed. https://pubmed.ncbi.nlm.nih.gov/12679331/. Accessed 5 Aug 2021
Carroll J, Ding S, Fearnley IM, Walker JE (2013) Post-translational modifications near the quinone binding site of mammalian complex I. J Biol Chem 288:24799–24808. https://doi.org/10.1074/jbc.M113.488106
Perdivara I, Deterding LJ, Przybylski M, Tomer KB (2010) Mass spectrometric identification of oxidative modifications of tryptophan residues in proteins: chemical artifact or post-translational modification? J Am Soc Mass Spectrom 21:1114–1117. https://doi.org/10.1016/j.jasms.2010.02.016
Sazanov LA (2015) A giant molecular proton pump: structure and mechanism of respiratory complex I. Nat Rev Mol Cell Biol 16:375–388. https://doi.org/10.1038/nrm3997
Dang Q-CL, Phan DH, Johnson AN et al (2020) Analysis of human mutations in the supernumerary subunits of complex I. Life 10:296. https://doi.org/10.3390/life10110296
Mimaki M, Wang X, McKenzie M et al (2012) Understanding mitochondrial complex I assembly in health and disease. Biochim Biophys Acta 1817:851–862. https://doi.org/10.1016/j.bbabio.2011.08.010
Unni S, Thiyagarajan S, Srinivas Bharath MM, Padmanabhan B (2019) Tryptophan oxidation in the UQCRC1 subunit of mitochondrial complex III (ubiquinol-cytochrome C reductase) in a mouse model of myodegeneration causes large structural changes in the complex: a molecular dynamics simulation study. Sci Rep 9:10694. https://doi.org/10.1038/s41598-019-47018-6
Sunitha B, Kumar M, Gowthami N et al (2020) Human muscle pathology is associated with altered phosphoprotein profile of mitochondrial proteins in the skeletal muscle. J Proteom 211:103556. https://doi.org/10.1016/j.jprot.2019.103556
Moser CC, Farid TA, Chobot SE, Dutton PL (2006) Electron tunneling chains of mitochondria. Biochim Biophys Acta 1757:1096–1109. https://doi.org/10.1016/j.bbabio.2006.04.015
Tocilescu MA, Zickermann V, Zwicker K, Brandt U (2010) Quinone binding and reduction by respiratory complex I. Biochim Biophys Acta 1797:1883–1890. https://doi.org/10.1016/j.bbabio.2010.05.009
Lenaz G, Fato R, Genova ML et al (2006) Mitochondrial Complex I: structural and functional aspects. Biochim Biophys Acta 1757:1406–1420. https://doi.org/10.1016/j.bbabio.2006.05.007
Jagatha B, Mythri RB, Shireen Vali MM, Bharath S (2008) Curcumin treatment alleviates the effects of glutathione depletion in vitro and in vivo: therapeutic implications for Parkinson’s disease explained via in silico studies. Free Radical Biol Med 44:907–917. https://doi.org/10.1016/j.freeradbiomed.2007.11.011
Mythri RB, Harish G, Dubey SK et al (2011) Glutamoyl diester of the dietary polyphenol curcumin offers improved protection against peroxynitrite-mediated nitrosative stress and damage of brain mitochondria in vitro: implications for Parkinson’s disease. Mol Cell Biochem 347:135–143. https://doi.org/10.1007/s11010-010-0621-4
Mythri RB, Veena J, Harish G et al (2011) Chronic dietary supplementation with turmeric protects against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-mediated neurotoxicity in vivo: implications for Parkinson’s disease. Br J Nutr 106:63–72. https://doi.org/10.1017/S0007114510005817
Shinomol GK, Bharath MMS, Muralidhara (2012) Neuromodulatory propensity of Bacopa monnieri leaf extract against 3-nitropropionic acid-induced oxidative stress: in vitro and in vivo evidences. Neurotox Res 22:102–114. https://doi.org/10.1007/s12640-011-9303-6
Shinomol GK, BharathMuralidhara MMS (2012) Pretreatment with Bacopa monnieri extract offsets 3-nitropropionic acid induced mitochondrial oxidative stress and dysfunctions in the striatum of prepubertal mouse brain. Can J Physiol Pharmacol 90:595–606. https://doi.org/10.1139/y2012-030
Shinomol GK, Mythri RB, Srinivas Bharath MM, Muralidhara (2012) Bacopa monnieri extract offsets rotenone-induced cytotoxicity in dopaminergic cells and oxidative impairments in mice brain. Cell Mol Neurobiol 32:455–465. https://doi.org/10.1007/s10571-011-9776-0
Shinomol GK, Raghunath N, Bharath MMS, Muralidhara (2013) Prophylaxis with Bacopa monnieri attenuates acrylamide induced neurotoxicity and oxidative damage via elevated antioxidant function. Cent Nerv Syst Agents Med Chem 13:3–12. https://doi.org/10.2174/1871524911313010003
Perez-Riverol Y, Bai J, Bandla C et al (2022) The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res 50:D543–D552. https://doi.org/10.1093/nar/gkab1038
Kang PT, Chen C-L, Lin P et al (2018) Mitochondrial complex I in the post-ischemic heart: reperfusion-mediated oxidative injury and protein cysteine sulfonation. J Mol Cell Cardiol 121:190–204. https://doi.org/10.1016/j.yjmcc.2018.07.244
Rhein VF, Carroll J, Ding S et al (2013) NDUFAF7 methylates arginine 85 in the NDUFS2 subunit of human complex I. J Biol Chem 288:33016–33026. https://doi.org/10.1074/jbc.M113.518803
Acknowledgements
This study was supported by the institutional funds allocated to the Department of Clinical Psychopharmacology and Neurotoxicology, NIMHANS. The technical help provided by Vismaya Meghalamane regarding Complex I proteomics experiments is gratefully acknowledged.
Funding
This study was supported by the National Institute of Mental Health and Neurosciences
Author information
Authors and Affiliations
Contributions
Y.C. carried out the experiments, analyzed the data and wrote the first draft of the manuscript. G.D. and V.G. contributed to the mass spectrometry experiments including data analysis. V.C. contributed to the Molecular Dynamics Simulation and preparation of related figures. NG contributed to proteomic data analysis and PTM analysis. V.V. supervised the research study. M.M.S.B. designed and supervised the study, analyzed the data, edited and prepared the final version of the manuscript. All the authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chithra, Y., Dey, G., Ghose, V. et al. Mitochondrial Complex I Inhibition in Dopaminergic Neurons Causes Altered Protein Profile and Protein Oxidation: Implications for Parkinson’s disease. Neurochem Res 48, 2360–2389 (2023). https://doi.org/10.1007/s11064-023-03907-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03907-x