Abstract
Epilepsy is a common and severe neurological disorder in which impaired glucose metabolism leads to changes in neuronal excitability that slow or promote the development of epilepsy. Leptin and adiponectin are important mediators regulating glucose metabolism in the peripheral and central nervous systems. Many studies have reported a strong association between epilepsy and these two adipokines involved in multiple signaling cascades and glucose metabolism. Due to the complex regulatory mechanisms between them and various signal activation networks, their role in epilepsy involves many aspects, including the release of inflammatory mediators, oxidative damage, and neuronal apoptosis. This paper aims to summarize the signaling pathways involved in leptin and adiponectin and the regulation of glucose metabolism from the perspective of the pathogenesis of epilepsy. In particular, we discuss the dual effects of leptin in epilepsy and the relationship between antiepileptic drugs and changes in the levels of these two adipokines. Clinical practitioners may need to consider these factors in evaluating clinical drugs. Through this review, we can better understand the specific involvement of leptin and adiponectin in the pathogenesis of epilepsy, provide ideas for further exploration, and bring about practical significance for the treatment of epilepsy, especially for the development of personalized treatment according to individual metabolic characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Adipokines, containing various bioactive peptides/proteins, immune molecules and inflammatory mediators, are secreted by adipose tissue and normally function through autocrine, paracrine and endocrine signaling [1]. Leptin and adiponectin, as two pivotal adipokines, act extensively in the central nervous system, and play an important role in the pathophysiology of different neurological diseases, including epilepsy [2,3,4,5,6].
Leptin, as a vital regulator in different physiopathologic processes, binds to leptin receptors and activates signaling pathways in the regulating different cellular functions to be neuroprotective. Although mice deficient in the mitochondrial manganese superoxide dismutase (MnSOD or SOD2) exhibit spontaneous seizures [7], leptin induces the production of MnSOD and the anti-apoptotic protein B-cell lymphoma-extra large (Bcl-XL), stabilizes the mitochondrial membrane potential and alleviates mitochondrial oxidative stress [8]. Mice deficient in leptin receptors are more prone to hippocampal damage caused by epilepsy, and intraventricular administration of leptin protects hippocampal neurons [8]. Electrophysiological and biochemical tests have shown that leptin has an anticonvulsant effect on pentylenetetrazol (PTZ) -induced generalized tonic–clonic convulsive seizure in the Wistar rat model. Leptin significantly increases the serum endogenous anticonvulsant agent galanin and glutathione (GSH). Besides, it decreases the expression level of malondialdehyde (MDA), which may be protective against oxidative damage [9].
Adiponectin is the 30 kDa adipocyte complement-related protein (Acrp30). Its receptors cover many biological functions. Adiponectin receptor 1 (AdipoR1) and receptor 2 (AdipoR2) have physiological correlations in metabolic processes. T-cadherin, a cadherin superfamily member, is a potent receptor for hexamer and adiponectin oligomers with high molecular weight [10,11,12]. Full-length adiponectin is cleaved by leukocyte esterase to form globular adiponectin (gAd) [13]. AdipoR1 has a high affinity for gAd, compared with AdipoR2 for full-length and gAd as an intermediate-affinity receptor [14]. Adiponectin transcription is regulated by Sirtuin 1/forkhead box protein O 1 (FoxO1) and peroxisome proliferator-activated receptors (PPARs) [15]. Adiponectin exerts a neuroprotective effect on brain damage in different regions through AdipoR1 [16,17,18,19,20] and, especially in the hippocampus, promotes neurogenesis through AdipoR1 [21] and directly affects synaptic function by AdipoR2 [22]. Adiponectin deficiency in mice on a high-fat diet results in increased seizure severity and pathological changes in the hippocampus [19].
Via bioinformatics technology, researchers used Gene Ontology (GO) and the Kyoto Encyclopedia of Genes and Genomes (KEGG) network to reveal that synapses play a crucial role in medial temporal lobe epilepsy (MTLE) [23]. It has been reported that the changes of postsynaptic glutamate receptor increase the excitability of hippocampal neural network, and 4-aminopyridine (4-AP) -induced epileptiform activity in hippocampal brain slices of rat in vitro model shows that alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)/N-methyl-D-aspartate (NMDA) ratio increases [24]. Using a cell model cultured with low magnesium, it has been observed that prolonged epileptiform activity increases reactive oxygen species (ROS) production in an NMDA receptor-dependent manner, further resulting in neuronal damage and apoptosis induced by epilepsy [25]. It has been described that oxidative damage in surgically resected brain tissue with epilepsy [26, 27]; conversely, oxidative damage may affect neuronal excitability and susceptibility to suffer from epilepsy [28,29,30].
Seizures and their potential effects on the development of the brain, especially for patients with clinically intractable epilepsy, may experience varying degrees of cognitive impairment, behavioral abnormalities, or psychiatric symptoms, all of which cause severe limitations in daily lives [31, 32]. However, many scholars have devoted to study the regulatory mechanism of leptin and adiponectin which has provided unique insights and opened a new perspective for the pathogenesis and treatment of epilepsy. This article mainly discusses the role of leptin and adiponectin in the genesis of epilepsy and their effects on antiepileptic drug treatment, in order to find an intervention to regulate signal transduction to control the progression of epilepsy and improve the quality of life in patients with epilepsy.
Epilepsy
Dysfunction in metabolic processes can bring about changes in neuronal excitability [33], promoting or alleviating seizure progression. Proteomic technology to screen for differential molecules associated with seizures has indicated that most of the affected proteins involve in energy metabolism and redox balance [34]. This article will discuss the correlation among various biological functions and complex signaling mechanisms of leptin and adiponectin, the way how these two adipokines regulate the metabolism and energy homeostasis and the pathological processes of epilepsy.
Febrile seizures(FS) is a common convulsive disease in children. Low-level leptin in cerebrospinal fluid (CSF) is related to the susceptibility to complex febrile seizures [35]. Chronic deficiency of leptin increases the susceptibility to seizures, severity, and possibility of suffering from generalized clonic and clonic-tonic seizures in PTZ-induced models [36]. However, current evidence has suggested that adiponectin is specifically expressed in different types of epilepsy. The logistic regression analysis has shown that a high serum adiponectin level is a significant risk factor for FS [37]. Inconsistently, serum adiponectin level reduces in adults with temporal lobe epilepsy (TLE) and in patients with refractory epilepsy [4, 38]. A study for 13 female patients has indicated that the plasma adiponectin levels are significantly increased within 24 h after primary or secondary generalized tonic–clonic seizures [39].
The pathogenesis of epilepsy starts from pathophysiological changes to the progression after seizures, including changes in voltage and transmitter-gated channels, intracellular signal cascades, synaptic connection, alteration in gene expression, abnormal protein production and activation or inhibition of the metabolic pathway, etc., which may be targeted indicators for the drug to inhibit epileptogenesis.
The Effects of Leptin on Epilepsy
The Signaling Cascade of Leptin
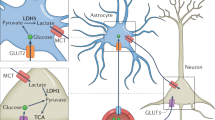
Leptin is a peptide hormone derived from adipocytes. Leptin receptors are expressed in both neonatal and adult hippocampal neurons, and through the blood–brain barrier (BBB) act in many regions of the central nervous system, where they involve in the regulation of energy balance, inflammatory processes, synapse formation and neurotrophic activity [9, 40,41,42,43]. Leptin binds to long-form receptors at the plasma membrane, to trigger multiple signaling cascades (Fig. 1), including Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling, phosphatidylinositol 3-kinase (PI3K)/protein kinase B(Akt)/FOXO1 signaling, and Src homology 2 domain-containing protein tyrosine phosphatase 2 (SHP2)- extracellular signal-regulated kinase (ERK) signaling.
The JAK-STAT pathway is a major signaling mechanism for various cytokines and growth factors. As a family of non-receptor tyrosine kinases, JAK activates and stimulates cell proliferation and apoptosis. After being phosphorylated by JAK, the substrate STAT dimerizes and crosses the nuclear envelope into the nucleus to regulate the expression of related genes [44]. In addition, receptors phosphorylated by JAKs recruit PI3K to activate the PI3K-Akt pathway. Akt phosphorylates target proteins through various downstream pathway to play a role in inhibiting apoptosis [45,46,47]. For example, Akt phosphorylates FoxO1, a transcription factor of the FoxO family, at multiple sites, resulting in the translocation of FoxO1 from the nucleus to the cytoplasm.
The Regulatory Mechanisms of Leptin in Glucose Metabolism
Glucose metabolism disorder affects the progression of epilepsy [48, 49]. Leptin, as an essential mediator of metabolic homeostasis, regulates glucose homeostasis both externally and centrally, and the peripheral targets are pancreatic β-cells. Depending on NMDA receptors, calcium/calmodulin-dependent kinase β (CaMKKβ) and AMP-activated protein kinase (AMPK), leptin increases cell membrane protein kinase A (PKA) activity, induces ATP-sensitive potassium (KATP) channels transport to the β-cells surface to inhibit glucose-stimulated insulin secretion, thereby increasing K+ conductance and causing β-cells hyperpolarization [50].
Studies have demonstrated increased glucose uptake in the brain after intravenous leptin administration in wild-type mice [51]. Leptin in neurons influences cellular glucose uptake through glucose transporters [52, 53]. Leptin also enhances lactate dehydrogenase A (LDHA) -dependent glucose perception in the hypothalamus to regulate glucose production in high-fat-fed rodents [54]. However, for 18 h food-deprived rats, microinjection of glucose in the hypothalamic paraventricular nucleus (PVN) can reduce plasma leptin levels [55]. The PI3K signaling pathway in hypothalamic neurons integrates leptin and insulin to coordinate systemic glucose and energy homeostasis [56]. Research has found that agouti-related peptide (AGRP) neurons play a role in the leptin regulation of energy balance and glucose homeostasis [40]. Potential mechanisms for its neurobiological effects include presynaptic enhancement of gamma-aminobutyric acid (GABA) neurotransmission and postsynaptic activation of adenosine triphosphate (ATP) -sensitive potassium channels. Continuous activation of leptin receptor neurons in the arcuate nucleus of the hypothalamus leads to impaired glucose tolerance [57]. Leptin receptors in the commissural nucleus of the tractus solitarius (cNTS) induce brain glucose retention (BGR) by enhancing hypoxic stimulation of carotid chemoreceptors [58].
Dual Roles of Leptin in Epilepsy
Leptin involves in energy metabolism through the PI3K-Akt-FoxO1 pathway in neurons [59]. However, the antiepileptic drug valproic acid (VPA) promotes the phosphorylation of Akt and FoxO1 [60]. Studies have shown that leptin activates the JAK/STAT and PI3K-Akt pathway and promotes neuronal survival by increasing the production of the antioxidant enzyme Mn-SOD and the anti-apoptotic protein Bcl-XL [8]. PI3K/Akt/Mechanistic target of rapamycin (mTOR) pathway may cause abnormal transduction of neuronal signal in epilepsy under pathological conditions [61,62,63].
The expression of Jak1, Stat1 and Stat3 in the hippocampal tissue of epileptic rats induced by lithium-pilocarpine increases [64]. Status epilepticus (SE) activates the JAK/STAT pathway [65], and selective inhibitors of the JAK/STAT pathway administered within 1 h after the onset of SE lead to transient suppression of STAT3 phosphorylation (pSTAT3) and a long-term reduction in the frequency of spontaneous seizures. In the study based on chromatin immunoprecipitation and chromatin immunoprecipitation followed by sequencing (ChIP-seq) [66], Lesiak et al. found that the suppressor of cytokine signaling 3 (SOCS3) is a direct target of the Cyclic AMP response-element binding protein (CREB) transcription factor.Leptin activates the Mitogen-activated protein kinase (MAPK) kinase (MEK)/ERK pathway and upregulates SOCS3 expression through the CREB transcription factor, thereby increasing synaptogenesis in hippocampal neurons [43]. The mechanism of leptin and STAT3 may depend on the interaction between SHP2 and SOCS3. After leptin receptors are activated, the conserved tyrosine residues on its tail are phosphorylated by JAK2 to promote the aggregation of downstream signaling proteins. Cytoplasmic tyrosine residue Tyr985 phosphorylates to combine with SHP2 which contains Src homology2 (SH2) domains and SOCS3. SHP2 binds phosphorylated Tyr985 and mediates the activation of ERK in cultured cells. SOCS3 mediates feedback inhibition of LepRb signaling by binding to Tyr985. SHP2 acts as a competitive and negative regulator in the processes of SOCS3's binding to Tyr985 associated with leptin receptors [67, 68]. SHP2 contains phosphatase domains and tyrosine phosphorylation sites. It plays a part in the regulation of cellular transduction pathway related to cytokine, growth factors and hormones, especially rat sarcoma (RAS)/MAPK and PI3K/AKT cascades. SHP2 dephosphorylates RAS and enhances its binding to the effector protein rapidly accelerated fibrosarcoma (RAF), thereby activating the downstream MEK/ERK signaling pathway [69]. MEK1 expression in the mouse brain not only leads to ERK activation to bring about spontaneous seizures, but also to phosphorylation of the transcription factor CREB [70]. Nguyen LH et al. [71] found that MEK inhibitor PD0325901 (mirdametinib) significantly decrease seizure activity in tuberous sclerosis complex (TSC) mouse models.
In addition to the abnormal release of neurotransmitters, epilepsy is closely related to the highly synchronized abnormal firing of neurons caused by abnormally transmembrane movement of ions. The changes in the structure and function of ion channels lead to excitatory regulation disorder to induce epilepsy. Leptin, however, plays an essential role in different epileptic models. Researchers have found that leptin counteracts the up-regulation of the protein level of the Zn (2 +)/Ca(2 +) signaling, which has a neuroprotective effect in the pilocarpine-induced neonatal Sprague–Dawley rat status epilepticus model [72] and inhibits the excitability of hippocampal neurons by activating Ca2+- and voltage-gated K+ channels of large conductance (BK channels) through PI3K [73]. The duration and incidence of focal seizures induced by 4-AP, an inhibitor of voltage-gated K+ channels, decrease after neocortical injection of leptin. Intranasal administration of leptin in mice delays the seizures of generalized convulsions induced by the chemical convulsant PTZ [74]. Leptin not only plays a potentially neuroprotective role by reducing cell damage related to SE induced by kainic acid (KA) [75], but also lowers the neuronal spiking in an in vitro epilepsy model and inhibits alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor-mediated synaptic transmission in the mouse hippocampus [74]. Ligand-gated ion channels, glutamate receptors mediate excitatory synaptic transmission in the central nervous system, and leptin directly affects glutamate neurotransmission in the hippocampus to inhibit seizures [74]. In hippocampal astrocytes of epileptic mice [76], pretreatment with leptin reduces the toxicity of excess glutamate to glial cells and plays a protective role against seizures. Leptin treatment improves the neurobehavioral abnormality generated by Flurothyl-induced recurrent seizures. Moreover, long-term treatment with leptin reverses the up-regulation of Beclin-1/Bcl-2 protein level and the down-regulation of Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIα) level [77]. Other evidence has shown that leptin reduces the expression of proinflammatory cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β) and interleukin 6 (IL-6) levels, suggesting that leptin may have an anti-inflammatory effect upon epileptic seizures [9].
Notably, studies have reported the proconvulsant activity of leptin. Intraventricular administration of leptin at a dose of 1 μg in a rat model with penicillin-induced epilepsy increases the mean frequency of epileptiform activity, but never changes its amplitude [78]. Electrophysiological studies have proven that inhibition of cannabinoids can mediate leptin's convulsant-stimulating activity [79]. Activating the cannabinoid receptors type 1 (CB1) mediates the anticonvulsant effect of cannabinoids [80]. Intraventricular injection of 7.5 μg CB1 agonist arachidonyl‐2‐chloroethylamide (ACEA) protects against penicillin-induced epileptoid activity. However, leptin blocks this effect and enhances the convulsant-promoting effect of [N‐(piperidine‐1‐yl)‐5‐(4‐iodophenyl)‐1‐(2,4‐dichlorophenyl)‐4‐methyl‐1H‐pyrazole‐3 carboxamide] (AM‐251), a cannabinoid CB1 receptor antagonist [79]. Moreover, leptin blocks glucocorticoid-mediated endocannabinoid release in the paraventricular nucleus of the hypothalamus through phosphodiesterase 3B-mediated reduction of intracellular cyclic adenosine monophosphate (cAMP) levels [81]. In addition, such changes may be related to the involvement of the neuronal nitric oxide synthase (NOS)/nitric oxide (NO) pathway in mediating the seizure-like activity in the processes mentioned above. nNOS increases γ-aminobutyric acid transaminase (GABA-T) activity and reduces brain GABA levels, and the NO produced may activate NMDA receptors [82]. Leptin increases NMDA (dual voltage and transmitter-gated channel) receptor-mediated synaptic currents and triggers N-methyl-D-aspartate receptor (NMDAR) -dependent Ca2+ influx [83]. Experimental research [84] has described that leptin exhibits dose-related proconvulsant activity with NMDA and KA, including decreasing latency and increasing symptoms.
Although leptin has shown inhibitory and neuroprotective activity against seizures in several epileptic models, it also increases epileptiform activity in other models under certain conditions. Reports on leptin's pro-convulsant and anticonvulsant effects have indicated specific roles of leptin in different epilepsy models and signaling pathways.
At present, antiepileptic drugs are still the mainly clinical treatment for epilepsy [85, 86]. Among children receiving long-term treatment with VPA, carbamazepine (CBZ) and lamotrigine (LTG), the serum leptin level remarkably increases in the VPA group [87]. Administration of antiepileptic drugs for at least 6 months for the over-6 age group with idiopathic epilepsy has shown VPA-treated children have higher leptin concentration and a lower ratio of soluble leptin receptor (SOB-R) to leptin [88]. Among children with idiopathic epilepsy or location-related idiopathic epilepsy in the monotherapy with VPA or topiramate (TPM) for at least 6 months [89], the leptin levels in the VPA group are higher than that in the TPM group. Some researchers believe that changes in leptin expression levels may also be one of the anticonvulsant mechanism of ketogenic diet (KD) [90, 91].
The Effects of Adiponectin on Epilepsy
The Involvement of Adiponectin in Signaling Cascade
Adiponectin is a hormone derived from adipocytes and is released into the circulation [13] in the form of full-length trimers, dimers (both of which are low molecular weight multimers), 18 or more high molecular weight multimers (HMW) [12, 92], and spherical moieties (gAD) [13]. It physiologically functions by activating downstream components of AMPK, P38-MAPK, c-Jun N-terminal kinase (JNK), and transcriptional regulatory nuclear factor-κB (NF-κB) signaling [14, 93,94,95]. However, the NAD + -dependent protein deacetylase SIRT1 and FoxO1-C enhancer binding protein α (EBPα) transcriptional complex affect the release of adiponectin [96, 97] (Fig. 2).
The Regulation of Adiponectin in Energy Metabolism
It has been reported that changes of adiponectin in the central nervous system (CNS) may affect glucose metabolism in hippocampal neurons [98]; Interestingly, the occurrence of epilepsy involves the disorder of glucose metabolism. With the medical device of (18) F-fluorodeoxyglucose-positron emission tomography (18F-FDG PET) imaging to analyze the glucose metabolic changes in medial temporal lobe epilepsy patients with hippocampal sclerosis (mTLE‐HS), low signals could be routinely observed in the temporal and extratemporal areas [99]. Wang J et al. simultaneously used resting‐state functional Magnetic Resonance Imaging (rs fMRI) and 18F-FDG PET to study the coupling changes between brain metabolism and functional activity in mTLE-HS patients and found that hypometabolism, the fractional amplitude of low frequency fluctuations (fALFF) and increased regional Homogeneity (ReHo) areas are usually associated with the generation and transmission of epileptiform activity. There is a high coupling between resting-state spontaneous neural activity and glucose metabolism [100].
Since glucose cannot freely enter the cell through the lipid bilayer structure of the cell membrane, the transport function of glucose transporters (GLUT) on the cell membrane is necessary to achieve intracellular glucose intake. Glucose is an important energy source for the central nervous system. GLUT1 is expressed in CNS endothelial cells (ECs) and uncoupled with glycolysis in a Notch-dependent manner, and GLUT1 deficiency in stationary adult ECs leads to severe seizures with neuronal loss and CNS inflammation [101, 102]. GLUT1 deficiency syndrome (GLUT1 DS) caused by GLUT1 deficiency indicates the types of variable focal and multiple local seizure, and electroencephalography (EEG) manifestations [103]. The 2-[N-(7-Nitrobenz-2-oxa-1,3-diazol-4-yl) amino]-2-deoxy-D-glucose (2-NBDG) method and glucose uptake colorimetric method have shown that seizures could dramatically reduce neuronal glucose uptake and GLUT-3 expression [104]. However, study on hippocampal neurons from cultured primary rats and hippocampal slices from mice [98] have demonstrated that adiponectin enhances glucose uptake, glycolysis rate, and ATP production through the AMPK -dependent mechanism.
The Regulatory Mechanisms of Adiponectin in Epilepsy
Apoptosis and inflammation induced by brain injury are pathogenic factors of epilepsy [105]; Nevertheless, adiponectin can protect cultured hippocampal neurons against KA-induced cytotoxicity, reduce reactive oxygen species, decrease apoptotic cell death, and inhibit KA-induced caspase-3 activation [106]. On the other hand, seizures also induce the production of inflammatory mediators, which trigger the activation of the NF-κB pathway to promote the progress of the disease in turn [107].
Adiponectin induces nuclear translocation of NF-κB p65 subunit and phosphorylation of MAPKs in dendritic cells [108]. Zhang et al. proposed that adiponectin triggers the proliferation of adult hippocampal neural stem/progenitor cells (hNSCs) through the p38MAPK/glycogen synthase kinase-3β (GSK-3β)/β-catenin cascade. P38MAPK inhibitor SB203580 not only eliminates this potentiation [109], but also reduces phosphorylated p38 (p-p38) in the PTZ-triggered rat epilepsy model [110], resulting in a decrease in caspase 3 level (Fig. 2). In addition, globular adiponectin plays anti-inflammatory and antioxidant roles in microglia through the AdipoR1/NF-κB signaling pathway [111].
P38 and JNK, another MAPK family member, are homologous protein-serine/threonine kinases, and selective targeting of JNK to P38 has been demonstrated as a potential therapeutic approach to epilepsy [112]. Tai and colleagues [113] found noticeable JNK overactivation in a TLE rat model induced by pilocarpine. The frequency of seizures obviously reduces with the use of a broad-spectrum nonspecific JNK inhibitor (SP600125), in contrast to the consequence for a nonspecific MAPK activator. Theselective inhibition of JNK-interacting protein 3 (JIP3) by lentivirus (LV-375JIP3-RNAi) attenuates the severity of seizure, consisting of reduced susceptibility of mice to the epileptogenic properties of KA, delayed first attack and decreased seizure duration. Apart from inhibiting JNK activation and neuronal apoptosis in the hippocampal CA3 region, underexpression of JIP3 has also been observed to delay the processes of PTZ-induced seizure firing [114]. Another concern is that neuronal damage after seizures is related to BBB leakage, and adiponectin maintains the integrity of the BBB and reduces the expression levels of vascular endothelial growth factor (VEGF), endothelial nitric oxide synthase (eNOS) and NF-κB in the hippocampus after KA-induced seizures in mice [115]. Notoriously, the BBB's integrity is critical in maintaining homeostasis and neuroprotection. We need more studies to confirm that reducing inflammatory stimulation by protecting the BBB may be a promising intervention or therapeutic strategy for epilepsy.
Antiepileptic drugs may be a potential factor affecting serum adipokine levels [116, 117]. Currently, VPA [118] is commonly used as a first-line antiepileptic drug in clinical practice, and its treatment time is negatively correlated with adiponectin level [119]. Clinical research has reported that adiponectin levels in children with idiopathic generalized epilepsy, obese children with idiopathic epilepsy, and adult epilepsy patients significantly decrease after valproate treatment [116, 119, 120]. Prospective evaluation of the long-term effects of the monotherapy of VPA and LTG on metabolic parameters in female epileptic patients for one year has found a remarkable decrease in adiponectin levels in the VPA group [121]. VPA-induced hypoadiponectinemia is significantly associated with weight gain and insulin resistance [119, 121]. The therapeutic concentration of VPA decreases adiponectin promoter activity in differentiated 3T3-L1 adipocytes, and inhibits gene expression of adiponectin in mature adipocytes [122].
Surprisingly, TPM, a novel antiepileptic drug, obviously decreases Leptin/Adiponectin (L/A) ratio and increases serum adiponectin level. Studies have shown that TPM increases energy metabolism and leads to weight loss in children with epilepsy [123]. The concentration of HMW adiponectin significantly increases in KD obese adolescents with no caloric restriction or a low-calorie diet for 6 months [124]. Treatment with antiepileptic therapy for 3 months for children with GLUT1 DS-resistant epilepsy aged 3–9 years [125], serum adiponectin level increases in the KD group (some of whom are also treated with other antiepileptic agents), compared with VPA monotherapy.
The specific adiponectin expression in different antiepileptic drugs may be bound up with multiple factors, including obesity, insulin resistance and molecular biology. With the in-depth study of clinical and basic experiments, we expect to deeply understand the specific mechanism of adiponectin to provide the theoretical basis for the clinically individualized treatment of epilepsy.
Concluding Remarks and Future Perspectives
The adverse consequences of epilepsy affect people of all ages, and persistent seizures lead to accidents and even death. The control and treatment of epilepsy are essential to improve the patients' quality of life and to reduce mortality. Peripheral endocrine and metabolic factors regulate seizure threshold and seizure-related pathology by acting on neurons in the central nervous system, triggering intracellular signaling pathways or modulating neuronal activity. The dual role of leptin in epilepsy has attracted researchers' particular attention. On the one hand, the leptin receptor activates related signaling pathways to alleviate seizures and play a neuroprotective role. On the other hand, studies have reported the proconvulsant activity of Leptin in different models of epilepsy. The complex dual effects of leptin in treating epilepsy have brought about a more significant challenge, and its role in epilepsy control and treatment needs to be further studied. The association of leptin and adiponectin with epilepsy highlights the important role of these two adipokines in the pathophysiology of epilepsy pathogenesis. A large amount of evidence is helpful to better understand the complex biological mechanisms of leptin and adiponectin, and to provide ideas and a theoretical basis for the development and clinical application of effective hormone modulators in dealing with epilepsy in the future. We still need to further explore the role of adipokine imbalance in the adjusting effect of signal transduction in the pathogenesis of epilepsy, so as to find new methods and preventive measures for epilepsy.
Data Availability
Not applicable.
References
Kahn D, Macias E, Zarini S, Garfield A, Zemski Berry K, MacLean P et al (2022) Exploring visceral and subcutaneous adipose tissue secretomes in human obesity: implications for metabolic disease. Endocrinology. https://doi.org/10.1210/endocr/bqac140
Cui Q, Zhang Y, Tian N, Yang J, Ya D, Xiang W et al (2022) Leptin promotes angiogenesis via pericyte STAT3 pathway upon intracerebral hemorrhage. Cells. https://doi.org/10.3390/cells11172755
Tu WJ, Qiu HC, Liu YK, Liu Q, Zeng X, Zhao J (2020) Elevated levels of adiponectin associated with major adverse cardiovascular and cerebrovascular events and mortality risk in ischemic stroke. Cardiovasc Diabetol 19(1):125. https://doi.org/10.1186/s12933-020-01096-3
Toscano ECB, Lessa JMK, Gonçalves AP, Rocha NP, Giannetti AV, de Oliveira GN et al (2019) Circulating levels of adipokines are altered in patients with temporal lobe epilepsy. Epilepsy Behav 90:137–141. https://doi.org/10.1016/j.yebeh.2018.11.023
Kuo YC, Wang IH, Rajesh R (2021) Use of leptin-conjugated phosphatidic acid liposomes with resveratrol and epigallocatechin gallate to protect dopaminergic neurons against apoptosis for Parkinson’s disease therapy. Acta Biomater 119:360–374. https://doi.org/10.1016/j.actbio.2020.11.015
He K, Nie L, Ali T, Wang S, Chen X, Liu Z et al (2021) Adiponectin alleviated Alzheimer-like pathologies via autophagy-lysosomal activation. Aging Cell 20(12):e13514. https://doi.org/10.1111/acel.13514
Liang LP, Waldbaum S, Rowley S, Huang TT, Day BJ, Patel M (2012) Mitochondrial oxidative stress and epilepsy in SOD2 deficient mice: attenuation by a lipophilic metalloporphyrin. Neurobiol Dis 45(3):1068–1076. https://doi.org/10.1016/j.nbd.2011.12.025
Guo Z, Jiang H, Xu X, Duan W, Mattson MP (2008) Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J Biol Chem 283(3):1754–1763. https://doi.org/10.1074/jbc.M703753200
Oztas B, Sahin D, Kir H, Eraldemir FC, Musul M, Kuskay S et al (2017) The effect of leptin, ghrelin, and neuropeptide-Y on serum Tnf-Α, Il-1β, Il-6, Fgf-2, galanin levels and oxidative stress in an experimental generalized convulsive seizure model. Neuropeptides 61:31–37. https://doi.org/10.1016/j.npep.2016.08.002
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M et al (2007) Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med 13(3):332–339. https://doi.org/10.1038/nm1557
Ruiz M, Ståhlman M, Borén J, Pilon M (2019) AdipoR1 and AdipoR2 maintain membrane fluidity in most human cell types and independently of adiponectin. J Lipid Res 60(5):995–1004. https://doi.org/10.1194/jlr.M092494
Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF (2004) T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proc Natl Acad Sci USA 101(28):10308–10313. https://doi.org/10.1073/pnas.0403382101
Waki H, Yamauchi T, Kamon J, Kita S, Ito Y, Hada Y et al (2005) Generation of globular fragment of adiponectin by leukocyte elastase secreted by monocytic cell line THP-1. Endocrinology 146(2):790–796. https://doi.org/10.1210/en.2004-1096
Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S et al (2003) Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423(6941):762–769. https://doi.org/10.1038/nature01705
Park HS, Lim JH, Kim MY, Kim Y, Hong YA, Choi SR et al (2016) Resveratrol increases AdipoR1 and AdipoR2 expression in type 2 diabetic nephropathy. J Transl Med 14(1):176. https://doi.org/10.1186/s12967-016-0922-9
Zhao W, Kong F, Gong X, Guo Z, Zhao L, Wang S (2021) Activation of AdipoR1 with rCTRP9 preserves BBB Integrity through the APPL1/AMPK/Nrf2 signaling pathway in ICH mice. Oxid Med Cell Longev 2021:2801263. https://doi.org/10.1155/2021/2801263
Song J, Choi SM, Whitcomb DJ, Kim BC (2017) Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis 8(10):e3102. https://doi.org/10.1038/cddis.2017.491
Xu N, Zhang Y, Doycheva DM, Ding Y, Zhang Y, Tang J et al (2018) Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology 133:415–428. https://doi.org/10.1016/j.neuropharm.2018.02.024
Lee EB, Warmann G, Dhir R, Ahima RS (2011) Metabolic dysfunction associated with adiponectin deficiency enhances kainic acid-induced seizure severity. J Neurosci 31(40):14361–14366. https://doi.org/10.1523/jneurosci.3171-11.2011
Song J, Kang SM, Kim E, Kim CH, Song HT, Lee JE (2015) Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: an in vivo and in vitro study. Cell Death Dis 6(8):e1844. https://doi.org/10.1038/cddis.2015.220
Liu B, Liu J, Wang JG, Liu CL, Yan HJ (2020) AdipoRon improves cognitive dysfunction of Alzheimer’s disease and rescues impaired neural stem cell proliferation through AdipoR1/AMPK pathway. Exp Neurol 327:113249. https://doi.org/10.1016/j.expneurol.2020.113249
Bloemer J, Pinky PD, Smith WD, Bhattacharya D, Chauhan A, Govindarajulu M et al (2019) Adiponectin knockout mice display cognitive and synaptic deficits. Front Endocrinol (Lausanne) 10:819. https://doi.org/10.3389/fendo.2019.00819
Li K, Wu C, Zhu Y, Yin W, Ren H, Yang X (2022) Construction and analysis of a competing endogenous RNA network associated with circRNAs dysregulated in medial temporal lobe epilepsy. Epileptic Disord 24(2):373–385. https://doi.org/10.1684/epd.2021.1403
Ergina JL, Amakhin DV, Postnikova TY, Soboleva EB, Zaitsev AV (2021) Short-term epileptiform activity potentiates excitatory synapses but does not affect intrinsic membrane properties of pyramidal neurons in the rat hippocampus in vitro. Biomedicines 9(10):1374. https://doi.org/10.3390/biomedicines9101374
Kovac S, Domijan AM, Walker MC, Abramov AY (2014) Seizure activity results in calcium- and mitochondria-independent ROS production via NADPH and xanthine oxidase activation. Cell Death Dis. 5(10):e1442. https://doi.org/10.1038/cddis.2014.390
Pecorelli A, Natrella F, Belmonte G, Miracco C, Cervellati F, Ciccoli L et al (2015) NADPH oxidase activation and 4-hydroxy-2-nonenal/aquaporin-4 adducts as possible new players in oxidative neuronal damage presents in drug-resistant epilepsy. Biochim Biophys Acta 1852(3):507–519. https://doi.org/10.1016/j.bbadis.2014.11.016
Rumià J, Marmol F, Sanchez J, Giménez-Crouseilles J, Carreño M, Bargalló N et al (2013) Oxidative stress markers in the neocortex of drug-resistant epilepsy patients submitted to epilepsy surgery. Epilepsy Res 107(1–2):75–81. https://doi.org/10.1016/j.eplepsyres.2013.08.020
Fulton RE, Pearson-Smith JN, Huynh CQ, Fabisiak T, Liang LP, Aivazidis S et al (2021) Neuron-specific mitochondrial oxidative stress results in epilepsy, glucose dysregulation and a striking astrocyte response. Neurobiol Dis 158:105470. https://doi.org/10.1016/j.nbd.2021.105470
MacMullin P, Hodgson N, Damar U, Lee HHC, Hameed MQ, Dhamne SC et al (2020) Increase in seizure susceptibility after repetitive concussion results from oxidative stress, parvalbumin-positive interneuron dysfunction and biphasic increases in glutamate/GABA ratio. Cereb Cortex 30(12):6108–6120. https://doi.org/10.1093/cercor/bhaa157
Prakash C, Mishra M, Kumar P, Kumar V, Sharma D (2019) Dehydroepiandrosterone alleviates oxidative stress and apoptosis in iron-induced epilepsy via activation of Nrf2/ARE signal pathway. Brain Res Bull 153:181–190. https://doi.org/10.1016/j.brainresbull.2019.08.019
Abe C, Denney D, Doyle A, Cullum M, Adams J, Perven G et al (2020) Comparison of psychiatric comorbidities and impact on quality of life in patients with epilepsy or psychogenic nonepileptic spells. Epilepsy Behav 102:106649. https://doi.org/10.1016/j.yebeh.2019.106649
Phuong TH, Houot M, Méré M, Denos M, Samson S, Dupont S (2021) Cognitive impairment in temporal lobe epilepsy: contributions of lesion, localization and lateralization. J Neurol 268(4):1443–1452. https://doi.org/10.1007/s00415-020-10307-6
Giménez-Cassina A, Martínez-François JR, Fisher JK, Szlyk B, Polak K, Wiwczar J et al (2012) BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron 74(4):719–730. https://doi.org/10.1016/j.neuron.2012.03.032
Grove RA, Madhavan D, Boone CHT, Braga CP, Papackova Z, Kyllo H et al (2020) Aberrant energy metabolism and redox balance in seizure onset zones of epileptic patients. J Proteomics 223:103812. https://doi.org/10.1016/j.jprot.2020.103812
Azab SF, Abdalhady MA, Almalky MA, Amin EK, Sarhan DT, Elhindawy EM et al (2016) Serum and CSF adiponectin, leptin, and interleukin 6 levels as adipocytokines in Egyptian children with febrile seizures: a cross-sectional study. Ital J Pediatr 42:38. https://doi.org/10.1186/s13052-016-0250-y
Erbayat-Altay E, Yamada KA, Wong M, Thio LL (2008) Increased severity of pentylenetetrazol induced seizures in leptin deficient ob/ob mice. Neurosci Lett 433(2):82–86. https://doi.org/10.1016/j.neulet.2007.12.051
Chen JR, Jin MF, Tang L, Liu YY, Ni H (2020) Acute phase serum leptin, adiponectin, interleukin-6, and visfatin are altered in chinese children with febrile seizures: a cross-sectional study. Front Endocrinol (Lausanne) 11:531. https://doi.org/10.3389/fendo.2020.00531
Ethemoglu O, Ay H, Koyuncu I, Gönel A (2018) Comparison of cytokines and prooxidants/antioxidants markers among adults with refractory versus well-controlled epilepsy: a cross-sectional study. Seizure 60:105–109. https://doi.org/10.1016/j.seizure.2018.06.009
Palmio J, Vuolteenaho K, Lehtimäki K, Nieminen R, Peltola J, Moilanen E (2016) CSF and plasma adipokines after tonic-clonic seizures. Seizure 39:10–12. https://doi.org/10.1016/j.seizure.2016.04.010
Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P et al (2018) Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556(7702):505–509. https://doi.org/10.1038/s41586-018-0049-7
Garfield AS, Shah BP, Burgess CR, Li MM, Li C, Steger JS et al (2016) Dynamic GABAergic afferent modulation of AgRP neurons. Nat Neurosci 19(12):1628–1635. https://doi.org/10.1038/nn.4392
Bland T, Sahin GS, Zhu M, Dillon C, Impey S, Appleyard SM et al (2019) USP8 Deubiquitinates the leptin receptor and is necessary for leptin-mediated synapse formation. Endocrinology 160(8):1982–1998. https://doi.org/10.1210/en.2019-00107
Sahin GS, Dhar M, Dillon C, Zhu M, Shiina H, Winters BD et al (2020) Leptin stimulates synaptogenesis in hippocampal neurons via KLF4 and SOCS3 inhibition of STAT3 signaling. Mol Cell Neurosci 106:103500. https://doi.org/10.1016/j.mcn.2020.103500
Lahiri S, Seidel R, Engelhard M, Becker CF (2010) Photocontrol of STAT6 dimerization and translocation. Mol Biosyst 6(12):2423–2429. https://doi.org/10.1039/c0mb00019a
Ye Z, Wang N, Xia P, Wang E, Yuan Y, Guo Q (2012) Delayed administration of parecoxib, a specific COX-2 inhibitor, attenuated postischemic neuronal apoptosis by phosphorylation Akt and GSK-3β. Neurochem Res 37(2):321–329. https://doi.org/10.1007/s11064-011-0615-y
Almajali B, Johan MF, Al-Wajeeh AS, Wan Taib WR, Ismail I, Alhawamdeh M et al (2022) Gene expression profiling and protein analysis reveal suppression of the C-myc oncogene and inhibition JAK/STAT and PI3K/AKT/mTOR signaling by thymoquinone in acute myeloid leukemia cells. Pharmaceuticals (Basel). https://doi.org/10.3390/ph15030307
Liu Y, Zhang Y, Jia K, Dong Y, Ma W (2015) Metformin inhibits the proliferation of A431 cells by modulating the PI3K/Akt signaling pathway. Exp Ther Med 9(4):1401–1406. https://doi.org/10.3892/etm.2015.2220
McDonald TS, Carrasco-Pozo C, Hodson MP, Borges K (2017) Alterations in cytosolic and mitochondrial [U-(13)C]glucose metabolism in a chronic epilepsy mouse model. eNeuro. https://doi.org/10.1523/eneuro.0341-16.2017
Sondhi V, Agarwala A, Pandey RM, Chakrabarty B, Jauhari P, Lodha R et al (2020) Efficacy of ketogenic diet, modified atkins diet, and low glycemic index therapy diet among children with drug-resistant epilepsy: a randomized clinical trial. JAMA Pediatr 174(10):944–951. https://doi.org/10.1001/jamapediatrics.2020.2282
Cochrane VA, Yang Z, Dell’Acqua ML, Shyng SL (2021) AKAP79/150 coordinates leptin-induced PKA signaling to regulate K(ATP) channel trafficking in pancreatic β-cells. J Biol Chem 296:100442. https://doi.org/10.1016/j.jbc.2021.100442
Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ (1997) Acute stimulation of glucose metabolism in mice by leptin treatment. Nature 389(6649):374–377. https://doi.org/10.1038/38717
Benomar Y, Naour N, Aubourg A, Bailleux V, Gertler A, Djiane J et al (2006) Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase- dependent mechanism. Endocrinology 147(5):2550–2556. https://doi.org/10.1210/en.2005-1464
Fuente-Martín E, García-Cáceres C, Granado M, de Ceballos ML, Sánchez-Garrido M, Sarman B et al (2012) Leptin regulates glutamate and glucose transporters in hypothalamic astrocytes. J Clin Invest 122(11):3900–3913. https://doi.org/10.1172/jci64102
Abraham MA, Rasti M, Bauer PV, Lam TKT (2018) Leptin enhances hypothalamic lactate dehydrogenase A (LDHA)-dependent glucose sensing to lower glucose production in high-fat-fed rats. J Biol Chem 293(11):4159–4166. https://doi.org/10.1074/jbc.RA117.000838
Brojeni MS, Nasseri F, Haghparast A, Eliassi A (2020) Paraventricular nucleus-microinjected glucose increases food intake in 18 h food-deprived rats: a central regulatory mechanism on serum ghrelin and leptin levels. Eur J Pharmacol 876:173073. https://doi.org/10.1016/j.ejphar.2020.173073
Fujikawa T, Choi YH, Yang DJ, Shin DM, Donato J Jr, Kohno D et al (2019) P110β in the ventromedial hypothalamus regulates glucose and energy metabolism. Exp Mol Med 51(4):1–9. https://doi.org/10.1038/s12276-019-0249-8
Balland E, Chen W, Tiganis T, Cowley MA (2019) Persistent leptin signaling in the arcuate nucleus impairs hypothalamic insulin signaling and glucose homeostasis in obese mice. Neuroendocrinology 109(4):374–390. https://doi.org/10.1159/000500201
Lemus M, Mojarro C, Montero S, Ramírez-Flores M, Torres-Magallanes J, Maturano-Melgoza A et al (2022) Leptin in the commissural nucleus of the tractus solitarius (cNTS) and anoxic stimulus in the carotid body chemoreceptors increases cNTS leptin signaling receptor and brain glucose retention in rats. Medicina (Kaunas). https://doi.org/10.3390/medicina58040550
Fukuda M, Jones JE, Olson D, Hill J, Lee CE, Gautron L et al (2008) Monitoring FoxO1 localization in chemically identified neurons. J Neurosci 28(50):13640–13648. https://doi.org/10.1523/jneurosci.4023-08.2008
Wu JB, Shih JC (2011) Valproic acid induces monoamine oxidase A via Akt/forkhead box O1 activation. Mol Pharmacol 80(4):714–723. https://doi.org/10.1124/mol.111.072744
Huang XY, Hu QP, Shi HY, Zheng YY, Hu RR, Guo Q (2021) Everolimus inhibits PI3K/Akt/mTOR and NF-kB/IL-6 signaling and protects seizure-induced brain injury in rats. J Chem Neuroanat 114:101960. https://doi.org/10.1016/j.jchemneu.2021.101960
Campion TJ 3rd, Sheikh IS, Smit RD, Iffland PH 2nd, Chen J, Junker IP et al (2022) Viral expression of constitutively active AKT3 induces CST axonal sprouting and regeneration, but also promotes seizures. Exp Neurol 349:113961. https://doi.org/10.1016/j.expneurol.2021.113961
Xiao Z, Peng J, Wu L, Arafat A, Yin F (2017) The effect of IL-1β on synaptophysin expression and electrophysiology of hippocampal neurons through the PI3K/Akt/mTOR signaling pathway in a rat model of mesial temporal lobe epilepsy. Neurol Res 39(7):640–648. https://doi.org/10.1080/01616412.2017.1312070
Feng X, Xiong W, Yuan M, Zhan J, Zhu X, Wei Z et al (2019) Down-regulated microRNA-183 mediates the Jak/Stat signaling pathway to attenuate hippocampal neuron injury in epilepsy rats by targeting Foxp1. Cell Cycle 18(22):3206–3222. https://doi.org/10.1080/15384101.2019.1671717
Grabenstatter HL, Del Angel YC, Carlsen J, Wempe MF, White AM, Cogswell M et al (2014) The effect of STAT3 inhibition on status epilepticus and subsequent spontaneous seizures in the pilocarpine model of acquired epilepsy. Neurobiol Dis 62:73–85. https://doi.org/10.1016/j.nbd.2013.09.003
Lesiak A, Pelz C, Ando H, Zhu M, Davare M, Lambert TJ et al (2013) A genome-wide screen of CREB occupancy identifies the RhoA inhibitors Par6C and Rnd3 as regulators of BDNF-induced synaptogenesis. PloS One 8(6):e64658. https://doi.org/10.1371/journal.pone.0064658
Tups A, Stöhr S, Helwig M, Barrett P, Krol E, Schachtner J et al (2012) Seasonal leptin resistance is associated with impaired signalling via JAK2-STAT3 but not ERK, possibly mediated by reduced hypothalamic GRB2 protein. J Comp Physiol B 182(4):553–567. https://doi.org/10.1007/s00360-011-0637-4
Barnes TM, Shah K, Allison MB, Steinl GK, Gordian D, Sabatini PV et al (2020) Identification of the leptin receptor sequences crucial for the STAT3-Independent control of metabolism. Mol Metab 32:168–175. https://doi.org/10.1016/j.molmet.2019.12.013
Shi ZQ, Yu DH, Park M, Marshall M, Feng GS (2000) Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol 20(5):1526–1536. https://doi.org/10.1128/mcb.20.5.1526-1536.2000
Nateri AS, Raivich G, Gebhardt C, Da Costa C, Naumann H, Vreugdenhil M et al (2007) ERK activation causes epilepsy by stimulating NMDA receptor activity. Embo j 26(23):4891–4901. https://doi.org/10.1038/sj.emboj.7601911
Nguyen LH, Leiser SC, Song D, Brunner D, Roberds SL, Wong M et al (2022) Inhibition of MEK-ERK signaling reduces seizures in two mouse models of tuberous sclerosis complex. Epilepsy Res 181:106890. https://doi.org/10.1016/j.eplepsyres.2022.106890
Ni H, Chen SH, Li LL, Jin MF (2018) Leptin treatment prevents long-term abnormalities in cognition, seizure threshold, hippocampal mossy fiber sprouting and ZnT3/CB-D28k expression in a rat developmental “twist” seizure model. Epilepsy Res 139:164–170. https://doi.org/10.1016/j.eplepsyres.2017.12.009
Shanley LJ, O’Malley D, Irving AJ, Ashford ML, Harvey J (2002) Leptin inhibits epileptiform-like activity in rat hippocampal neurones via PI 3-kinase-driven activation of BK channels. J Physiol 545(3):933–944. https://doi.org/10.1113/jphysiol.2002.029488
Xu L, Rensing N, Yang XF, Zhang HX, Thio LL, Rothman SM et al (2008) Leptin inhibits 4-aminopyridine- and pentylenetetrazole-induced seizures and AMPAR-mediated synaptic transmission in rodents. J Clin Invest 118(1):272–280. https://doi.org/10.1172/jci33009
Obeid M, Frank J, Medina M, Finckbone V, Bliss R, Bista B et al (2010) Neuroprotective effects of leptin following kainic acid-induced status epilepticus. Epilepsy Behav 19(3):278–283. https://doi.org/10.1016/j.yebeh.2010.07.023
Jayaram B, Khan RS, Kastin AJ, Hsuchou H, Wu X, Pan W (2013) Protective role of astrocytic leptin signaling against excitotoxicity. J Mol Neurosci 49(3):523–530. https://doi.org/10.1007/s12031-012-9924-0
Li LL, Li YC, Zhao DJ, Jin MF, Ni H (2018) Leptin-regulated autophagy plays a role in long-term neurobehavioral injury after neonatal seizures and the regulation of zinc/cPLA2 and CaMK II signaling in cerebral cortex. Epilepsy Res 146:103–111. https://doi.org/10.1016/j.eplepsyres.2018.07.023
Aslan A, Yildirim M, Ayyildiz M, Güven A, Agar E (2010) Interaction of leptin and nitric oxide pathway on penicillin-induced epileptiform activity in rats. Brain Res 1321:117–124. https://doi.org/10.1016/j.brainres.2010.01.054
Arslan G, Alici SK, Ayyildiz M, Agar E (2013) The role of CB1-receptors in the proconvulsant effect of leptin on penicillin-induced epileptiform activity in rats. CNS Neurosci Ther 19(4):222–228. https://doi.org/10.1111/cns.12075
Luszczki JJ, Czuczwar P, Cioczek-Czuczwar A, Czuczwar SJ (2006) Arachidonyl-2′-chloroethylamide, a highly selective cannabinoid CB1 receptor agonist, enhances the anticonvulsant action of valproate in the mouse maximal electroshock-induced seizure model. Eur J Pharmacol 547(1–3):65–74. https://doi.org/10.1016/j.ejphar.2006.07.037
Malcher-Lopes R, Di S, Marcheselli VS, Weng FJ, Stuart CT, Bazan NG et al (2006) Opposing crosstalk between leptin and glucocorticoids rapidly modulates synaptic excitation via endocannabinoid release. J Neurosci 26(24):6643–6650. https://doi.org/10.1523/jneurosci.5126-05.2006
Vega Rasgado LA, Reyes GC, Vega DF (2018) Role of nitric oxide synthase on brain GABA transaminase activity and GABA levels. Acta Pharm 68(3):349–359. https://doi.org/10.2478/acph-2018-0022
Wu Y, Fortin DA, Cochrane VA, Chen PC, Shyng SL (2017) NMDA receptors mediate leptin signaling and regulate potassium channel trafficking in pancreatic β-cells. J Biol Chem 292(37):15512–15524. https://doi.org/10.1074/jbc.M117.802249
Lynch JJ 3rd, Shek EW, Castagné V, Mittelstadt SW (2010) The proconvulsant effects of leptin on glutamate receptor-mediated seizures in mice. Brain Res Bull 82(1–2):99–103. https://doi.org/10.1016/j.brainresbull.2010.02.003
Pong AW, Ross J, Tyrlikova I, Giermek AJ, Kohli MP, Khan YA et al (2022) Epilepsy: expert opinion on emerging drugs in phase 2/3 clinical trials. Expert Opin Emerg Drugs 27(1):75–90. https://doi.org/10.1080/14728214.2022.2059464
Krauss GL, Klein P, Brandt C, Lee SK, Milanov I, Milovanovic M et al (2020) Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol 19(1):38–48. https://doi.org/10.1016/s1474-4422(19)30399-0
Hamed SA, Fida NM, Hamed EA (2009) States of serum leptin and insulin in children with epilepsy: risk predictors of weight gain. Eur J Paediatr Neurol 13(3):261–268. https://doi.org/10.1016/j.ejpn.2008.05.005
Rauchenzauner M, Haberlandt E, Scholl-Bürgi S, Karall D, Schoenherr E, Tatarczyk T et al (2008) Effect of valproic acid treatment on body composition, leptin and the soluble leptin receptor in epileptic children. Epilepsy Res 80(2–3):142–149. https://doi.org/10.1016/j.eplepsyres.2008.03.017
Çiçek NP, Kamaşak T, Serin M, Okten A, Alver A, Cansu A (2018) The effects of valproate and topiramate use on serum insulin, leptin, neuropeptide Y and ghrelin levels in epileptic children. Seizure 58:90–95. https://doi.org/10.1016/j.seizure.2018.03.013
Ellenbroek JH, van Dijck L, Töns HA, Rabelink TJ, Carlotti F, Ballieux BE et al (2014) Long-term ketogenic diet causes glucose intolerance and reduced β- and α-cell mass but no weight loss in mice. Am J Physiol Endocrinol Metab 306(5):E552–E558. https://doi.org/10.1152/ajpendo.00453.2013
Thio LL, Erbayat-Altay E, Rensing N, Yamada KA (2006) Leptin contributes to slower weight gain in juvenile rodents on a ketogenic diet. Pediatr Res 60(4):413–417. https://doi.org/10.1203/01.pdr.0000238244.54610.27
Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T et al (2003) Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin Implications fpr metabolic regulation and bioactivity. J Biol Chem 278(11):9073–85. https://doi.org/10.1074/jbc.M207198200
Al-Anazi A, Parhar R, Saleh S, Al-Hijailan R, Inglis A, Al-Jufan M et al (2018) Intracellular calcium and NF-(k)B regulate hypoxia-induced leptin, VEGF, IL-6 and adiponectin secretion in human adipocytes. Life Sci 212:275–284. https://doi.org/10.1016/j.lfs.2018.10.014
Lindfors S, Polianskyte-Prause Z, Bouslama R, Lehtonen E, Mannerla M, Nisen H et al (2021) Adiponectin receptor agonist AdipoRon ameliorates renal inflammation in diet-induced obese mice and endotoxin-treated human glomeruli ex vivo. Diabetologia 64(8):1866–1879. https://doi.org/10.1007/s00125-021-05473-9
Yang W, Yuan W, Peng X, Wang M, Xiao J, Wu C et al (2019) PPAR γ/Nnat/NF-κB Axis Involved in Promoting Effects of Adiponectin on Preadipocyte Differentiation. Mediators Inflamm 2019:5618023. https://doi.org/10.1155/2019/5618023
Picard F, Kurtev M, Chung N, Topark-Ngarm A, Senawong T, Machado De Oliveira R et al (2004) Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 429(6993):771–6. https://doi.org/10.1038/nature02583
Qiao L, Shao J (2006) SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem 281(52):39915–39924. https://doi.org/10.1074/jbc.M607215200
Cisternas P, Martinez M, Ahima RS, William Wong G, Inestrosa NC (2019) Modulation of Glucose metabolism in hippocampal neurons by adiponectin and resistin. Mol Neurobiol 56(4):3024–3037. https://doi.org/10.1007/s12035-018-1271-x
Chassoux F, Artiges E, Semah F, Desarnaud S, Laurent A, Landre E et al (2016) Determinants of brain metabolism changes in mesial temporal lobe epilepsy. Epilepsia 57(6):907–919. https://doi.org/10.1111/epi.13377
Wang J, Shan Y, Dai J, Cui B, Shang K, Yang H et al (2020) Altered coupling between resting-state glucose metabolism and functional activity in epilepsy. Ann Clin Transl Neurol 7(10):1831–1842. https://doi.org/10.1002/acn3.51168
Veys K, Fan Z, Ghobrial M, Bouché A, García-Caballero M, Vriens K et al (2020) Role of the GLUT1 glucose transporter in postnatal CNS angiogenesis and blood-brain barrier integrity. Circ Res 127(4):466–482. https://doi.org/10.1161/circresaha.119.316463
Pervaiz I, Zahra FT, Mikelis CM, Al-Ahmad AJ (2022) An in vitro model of glucose transporter 1 deficiency syndrome at the blood-brain barrier using induced pluripotent stem cells. J Neurochem 162(6):483–500. https://doi.org/10.1111/jnc.15684
Pong AW, Geary BR, Engelstad KM, Natarajan A, Yang H, De Vivo DC (2012) Glucose transporter type I deficiency syndrome: epilepsy phenotypes and outcomes. Epilepsia 53(9):1503–1510. https://doi.org/10.1111/j.1528-1167.2012.03592.x
Peng W, Liu X, Tan C, Zhou W, Jiang J, Zhou X et al (2021) Zinc-α2-glycoprotein relieved seizure-Induced neuronal glucose uptake impairment via insulin-like growth factor 1 receptor-regulated glucose transporter 3 expression. J Neurochem 157(3):695–709. https://doi.org/10.1111/jnc.15254
Kwon YS, Pineda E, Auvin S, Shin D, Mazarati A, Sankar R (2013) Neuroprotective and antiepileptogenic effects of combination of anti-inflammatory drugs in the immature brain. J Neuroinflammation 10:30. https://doi.org/10.1186/1742-2094-10-30
Qiu G, Wan R, Hu J, Mattson MP, Spangler E, Liu S et al (2011) Adiponectin protects rat hippocampal neurons against excitotoxicity. Age (Dordr) 33(2):155–165. https://doi.org/10.1007/s11357-010-9173-5
He C, Su C, Zhang W, Zhou Q, Shen X, Yang J et al (2021) Modulatory potential of LncRNA Zfas1 for inflammation and neuronal apoptosis in temporal lobe epilepsy. Yonsei Med J 62(3):215–223. https://doi.org/10.3349/ymj.2021.62.3.215
Jung MY, Kim HS, Hong HJ, Youn BS, Kim TS (2012) Adiponectin induces dendritic cell activation via PLCγ/JNK/NF-κB pathways, leading to Th1 and Th17 polarization. J Immunol 188(6):2592–2601. https://doi.org/10.4049/jimmunol.1102588
Zhang D, Guo M, Zhang W, Lu XY (2011) Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3β (GSK-3β)/β-catenin signaling cascade. J Biol Chem 286(52):44913–44920. https://doi.org/10.1074/jbc.M111.310052
Huang Q, Liu X, Wu Y, Liao Y, Huang Y, Wei X et al (2017) P38 MAPK pathway mediates cognitive damage in pentylenetetrazole-induced epilepsy via apoptosis cascade. Epilepsy Res 133:89–92. https://doi.org/10.1016/j.eplepsyres.2017.04.012
Nicolas S, Cazareth J, Zarif H, Guyon A, Heurteaux C, Chabry J et al (2017) Globular adiponectin limits microglia pro-inflammatory phenotype through an AdipoR1/NF-κB signaling pathway. Front Cell Neurosci 11:352. https://doi.org/10.3389/fncel.2017.00352
Zhuo ZH, Sun YZ, Jin PN, Li FY, Zhang YL, Wang HL (2016) Selective targeting of MAPK family kinases JNK over p38 by rationally designed peptides as potential therapeutics for neurological disorders and epilepsy. Mol Biosyst 12(8):2532–2540. https://doi.org/10.1039/c6mb00297h
Tai TY, Warner LN, Jones TD, Jung S, Concepcion FA, Skyrud DW et al (2017) Antiepileptic action of c-Jun N-terminal kinase (JNK) inhibition in an animal model of temporal lobe epilepsy. Neuroscience 349:35–47. https://doi.org/10.1016/j.neuroscience.2017.02.024
Wang Z, Chen Y, Lü Y, Chen X, Cheng L, Mi X et al (2015) Effects of JIP3 on epileptic seizures: evidence from temporal lobe epilepsy patients, kainic-induced acute seizures and pentylenetetrazole-induced kindled seizures. Neuroscience 300:314–324. https://doi.org/10.1016/j.neuroscience.2015.05.008
Jeon BT, Shin HJ, Kim JB, Kim YK, Lee DH, Kim KH et al (2009) Adiponectin protects hippocampal neurons against kainic acid-induced excitotoxicity. Brain Res Rev 61(2):81–88. https://doi.org/10.1016/j.brainresrev.2009.05.002
Meral C, Cekmez F, Vurucu S, Tascılar E, Pirgon O, Canpolat FE et al (2011) New adipocytokines (vaspin, apelin, visfatin, adiponectin) levels in children treated with valproic acid. Eur Cytokine Netw 22(2):118–122. https://doi.org/10.1684/ecn.2011.0284
Aksoy A, Sönmez FM, Deger O, Hosver I, Karagüzel G (2011) The effects of antiepileptic drugs on the relationships between leptin levels and bone turnover in prepubertal children with epilepsy. J Pediatr Endocrinol Metab 24(9–10):703–708. https://doi.org/10.1515/jpem.2011.019
Marson A, Burnside G, Appleton R, Smith D, Leach JP, Sills G et al (2021) The SANAD II study of the effectiveness and cost-effectiveness of valproate versus levetiracetam for newly diagnosed generalised and unclassifiable epilepsy: an open-label, non-inferiority, multicentre, phase 4, randomised controlled trial. Lancet 397(10282):1375–1386. https://doi.org/10.1016/s0140-6736(21)00246-4
Aly RH, Amr NH, Saad WE, Megahed AA (2015) Insulin resistance in patients on valproic acid: relation to adiponectin. Acta Neurol Scand 131(3):169–175. https://doi.org/10.1111/ane.12313
Nisha Y, Bobby Z, Wadwekar V (2018) Biochemical derangements related to metabolic syndrome in epileptic patients on treatment with valproic acid. Seizure 60:57–60. https://doi.org/10.1016/j.seizure.2018.06.003
Sidhu HS, Srinivas R, Sadhotra A (2017) Evaluate the effects of long-term valproic acid treatment on metabolic profiles in newly diagnosed or untreated female epileptic patients: a prospective study. Seizure 48:15–21. https://doi.org/10.1016/j.seizure.2017.03.007
Qiao L, Schaack J, Shao J (2006) Suppression of adiponectin gene expression by histone deacetylase inhibitor valproic acid. Endocrinology 147(2):865–874. https://doi.org/10.1210/en.2005-1030
Li HF, Zou Y, Xia ZZ, Gao F, Feng JH, Yang CW (2009) Effects of topiramate on weight and metabolism in children with epilepsy. Acta Paediatr 98(9):1521–1525. https://doi.org/10.1111/j.1651-2227.2009.01349.x
Partsalaki I, Karvela A, Spiliotis BE (2012) Metabolic impact of a ketogenic diet compared to a hypocaloric diet in obese children and adolescents. J Pediatr Endocrinol Metab 25(7–8):697–704. https://doi.org/10.1515/jpem-2012-0131
Chyra M, Roczniak W, Świętochowska E, Dudzińska M, Oświęcimska J (2022) The Effect of the ketogenic diet on adiponectin, omentin and vaspin in children with drug-resistant epilepsy. Nutrients 14(3):479. https://doi.org/10.3390/nu14030479
Acknowledgements
All authors are grateful to the Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine for support.
Funding
This work was supported by the Youth Natural Science Foundation of Zhangjiagang TCM Hospital Affiliated to Nanjing University of Chinese Medicine (ZZYQ2009), Zhangjiagang City Science and Technology Planning project (ZKS2125) and Suzhou Medical Health Science and technology innovation project (SKYD2022047).
Author information
Authors and Affiliations
Contributions
YS, YS and YC completed initial draft of the manuscript and figures. HG and YW collected literature information. All authors revised and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have declared that no competing interest exists.
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shan, Y., Chen, Y., Gu, H. et al. Regulatory Basis of Adipokines Leptin and Adiponectin in Epilepsy: from Signaling Pathways to Glucose Metabolism. Neurochem Res 48, 2017–2028 (2023). https://doi.org/10.1007/s11064-023-03891-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-023-03891-2