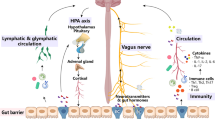
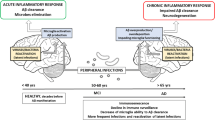
Abstract
The late onset neuropathologies, including Alzheimer’s disease and Parkinson’s disease, have become increasingly prevalent. Their causation has been linked to genetics, gut microbiota dysbiosis (gut dysbiosis), autoimmune diseases, pathogens and exposures to neurotoxins. An alternative explanatory hypothesis is provided for their pathogenesis. Virtually everyone has pervasive daily exposures to neurotoxins, through inhalation, skin contact, direct blood transmission and through the gastrointestinal tract by ingestion. As a result, every individual has substantial and fluctuating neurotoxin blood levels. Two major barriers to neurotoxin entry into the central nervous system are the blood–brain barrier and the intestinal wall, in the absence of gut dysbiosis. Inflammation from gut dysbiosis, induced by antibiotic usage, can increase the intestinal wall permeability for neurotoxins to reach the bloodstream, and also increase the blood–brain barrier permeability to neurotoxins. Gut dysbiosis, including gut dysbiosis caused by antibiotic treatments, is an especially high risk for neurotoxin entry into the brain to cause late onset neuropathologies. Gut dysbiosis has far-reaching immune system and central nervous system effects, and even a transient gut dysbiosis can act in combination with neurotoxins, such as aluminum, mercury, lead, arsenic, cadmium, selenium, manganese, organophosphate pesticides and organochlorines, to reach neurotoxin blood levels that can initiate a late onset neuropathology, depending on an individual’s age and genetic vulnerability.



Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
Abbreviations
- SNCA:
-
Synuclein, Alpha
- GI:
-
Gastrointestinal
- CNS:
-
Central nervous system
- HPA:
-
Hypothalamic–pituitary–adrenal
- GABA:
-
γ-Aminobutyric acid
- IgG:
-
Immunoglobulin G
- CD4:
-
Cluster of differentiation 4
- TH1:
-
Helper 1 T cells
- TH17:
-
Helper 17 T cells
- IL-1:
-
Interleukin-1
- IL-6:
-
Interleukin-6
- IL-17:
-
Interleukin-17
- TNF-α:
-
Tumor necrosis factor-α
- eNOS:
-
Endothelial nitric oxide synthase
- BBB:
-
Blood–brain barrier
- ROS:
-
Reactive oxygen species
- GSH:
-
Glutathione
- NO:
-
Nitric oxide
- NOS:
-
Nitric oxygen synthase
- iNOS:
-
Inducible nitric oxygen synthase
- DDT:
-
Dichlorodiphenyltrichloroethane
- DDE:
-
Dichlorodiphenyldichloroethylene
References
Morris G, Puri BK, Frye RE (2017) The putative role of environmental aluminum in the development of chronic neuropathology in adults and children. How strong is the evidence and what could be the mechanisms involved? Metab Brain Dis 32(5):1335–1335
Alasfar RH, Isaifan RJ (2021) Aluminum environmental pollution: the silent killer. Environ Sci Pollut Res Int 28(33):44587–44597. https://doi.org/10.1007/s11356-021-14700-0
Cryan JF, O’riordan KJ, Cowan CSM, Sandhu KV, Bastiaanssen TFS, Boehme M, Codagnone MG et al (2019) The microbiota-gut-brain axis. Physiol Rev 99:1877–2013
Zhu S, Jiang Y, Xu K, Cui M, Ye W, Zhao G, Jin L, Chen X (2020) The progress of gut microbiome research related to brain disorders. J Neuroinflammation 17(1):25. https://doi.org/10.1186/s12974-020-1705-z
Skjærbæk C, Knudsen K, Horsager J, Borghammer P (2021) Gastrointestinal dysfunction in Parkinson’s disease. J Clin Med 10(3):493. https://doi.org/10.3390/jcm10030493
Liu B, Fang F, Pedersen NL, Tillander A, Ludvigsson JF, Ekbom A, Svenningsson P, Chen H, Wirdefeldt K (2017) Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 88(21):1996–2002. https://doi.org/10.1212/WNL.0000000000003961
Van Den Berge N, Ferreira N, Gram H, Mikkelsen TW, Alstrup AKO, Casadei N, Tsung-Pin P, Riess O, Nyengaard JR, Tamgüney G, Jensen PH, Borghammer P (2019) Evidence for bidirectional and trans-synaptic parasympathetic and sympathetic propagation of alpha-synuclein in rats. Acta Neuropathol 138(4):535–550. https://doi.org/10.1007/s00401-019-02040-w
Rolli-Derkinderen M, Leclair-Visonneau L, Bourreille A, Coron E, Neunlist M, Derkinderen P (2020) Is Parkinson’s disease a chronic low-grade inflammatory bowel disease? J Neurol 267:2207–2213. https://doi.org/10.1007/s00415-019-09321-0
Ramirez J, Guarner F, Bustos Fernandez L, Maruy A, Sdepanian VL, Cohen H (2020) Antibiotics as major disruptors of gut microbiota. Front Cell Infect Microbiol 10:572912. https://doi.org/10.3389/fcimb.2020.572912
Antonini M, Conte M, Sorini C, Falcone M (2019) How the interplay between the commensal microbiota, gut barrier integrity, and mucosal immunity regulates brain autoimmunity. Front Immunol 10:1937
Lau WL, Savoj J, Nakata MB, Vaziri ND (2018) Altered microbiome in chronic kidney disease: systemic effects of gut-derived uremic toxins. Clin Sci 132(5):509–522
Obrenovich MEM (2018) Leaky gut, leaky brain? Microorganisms 6(4):107
Coelho-Santos V, Shih AY (2020) Postnatal development of cerbrovascular structure and neurogliovascular unit. Wiley Interdiscip Rev Dev Biol 9(2):e363
Galea I (2021) The blood-brain barrier in systemic infection and inflammation. Cell Mol Immunol. https://doi.org/10.1038/s41423-021-00757-x
Profaci CP, Munji RN, Pulido RS, Daneman R (2020) The blood-brain barrier in health and disease: important unanswered questions. J Exp Med 217(4):e20190062. https://doi.org/10.1084/jem.20190062
Hussain B, Fang C, Chang J (2021) Blood-brain barrier breakdown: an emerging biomarker of cognitive impairment in normal aging and dementia. Front Neurosci 15:688090. https://doi.org/10.3389/fnins.2021.688090
Kern JK, Geier DA, Homme KG, King PG, Bjorklund G, Chirumbolo S, Geier MR (2017) Developmental neurotoxicants and the vulnerable male brain: a systematic review of suspected neurotoxicants that disproportionally affect males. Acta Neurobiol Exp 77(4):269–296
Gershon MD, Margolis KG (2021) The gut, its microbiome, and the brain: connections and communications. J Clin Invest 131(18):e143768. https://doi.org/10.1172/JCI143768
Jacobson A, Yang D, Vella M, Chiu IM (2021) The intestinal neuro-immune axis: crosstalk between neurons, immune cells, and microbes. Mucosal Immunol 14(3):555–565. https://doi.org/10.1038/s41385-020-00368-1
Janakiraman M, Krishnamoorthy G (2018) Emerging role of diet and microbiota interactions in neuroinflammation. Front Immunol 9:2067
Eshraghi RS, Davies C, Iyengar R, Perez L, Mittal R, Eshraghi AA (2021) Gut-Induced inflammation during development may compromise the blood-brain barrier and predispose to autism spectrum disorder. J Clin Med 10(1):27. https://doi.org/10.3390/jcm10010027
Fukui H (2016) Increased intestinal permeability and decrease barrier function: does it really influence the risk of inflammation? Inflamm Intest Dis 1(3):135–145
Igbokwe IO, Igwenagu E, Igbokwe NA (2019) Aluminum toxicosis; a review of toxic actions and effects. Interdiscip Toxicol 12(2):45–70
Tietz T, Lenzner A, Kolbaum AE, Zellmer S, Riebeling C, Gürtler R, Jung C, Kappenstein O, Tentschert J, Giulbudagian M, Merkel S, Pirow R, Lindtner O, Tralau T, Schäfer B, Laux P, Greiner M, Lampen A, Luch A, Wittkowski R, Hensel A (2019) Aggregated aluminium exposure: risk assessment for the general population. Arch Toxicol 93(12):3503–3521. https://doi.org/10.1007/s00204-019-02599-z
Bondy SC (2016) Low levels of aluminum can lead to behavioral and morphological changes associated with Alzheimer’s disease and age-related neurodegeneration. Neurotoxicology 52:222–229. https://doi.org/10.1016/j.neuro.2015.12.002
Skalny AV, Aschner M, Jiang Y, Gluhcheva YG, Tizabi Y, Lobinski R, Tinkov AA (2021) Molecular mechanisms of aluminum neurotoxicity: update on adverse effects and therapeutic strategies. Adv Neurotoxicol 5:1–34. https://doi.org/10.1016/bs.ant.2020.12.001
Tremblay MÈ (2021) Microglial functional alteration and increased diversity in the challenged brain: insights into novel targets for intervention. Brain Behav Immun Health 16:100301. https://doi.org/10.1016/j.bbih.2021.100301
Anderson SR, Vetter ML (2019) Developmental roles of microglia: a window into mechanisms of disease. Dev Dyn 248(1):98–117. https://doi.org/10.1002/dvdy.1
Hoeijmakers L, Heinen Y, van Dam AM, Lucassen PJ, Korosi A (2016) Microglial priming and Alzheimer’s disease: a possible role for (early) immune challenges and epigenetics? Front Hum Neurosci 10:398
Edmonson CA, Ziats MN, Rennert OM (2016) A non-inflammatory role for microglia in autism spectrum disorders. Front Neurol 7:9
Nutma E, van Gent D, Amor S, Peferoen LAN (2020) Astrocyte and oligodendrocyte cross-talk in the central nervous system. Cells 9(3):600. https://doi.org/10.3390/cells9030600
Verkhratsky A, Parpura V (2016) Astrogliopathology in neurological, neurodevelopmental and psychiatric disorders. Neurobiol Dis 85:254–261
Khakh BS, Sofroniew MV (2015) Diversity of astrocyte functions and phenotypes in neural circuits. Nat Neurosci 18(7):942–952
Siblerud R, Mutter J, Moore E, Naumann J, Walach H (2019) A hypothesis and evidence that mercury may be an etiological factor in Alzheimer’s disease. Int J Environ Res Public Health 16(24):5152
Genchi G, Sinicropi MS, Carocci A, Lauria G, Catalono A (2017) Mercury exposure and heart diseases. Int J Environ Res Public Health 14(1):74
Drevnick PE, Lamborg CH, Horgan MJ (2015) Increase in mercury in Pacific yellow fin tuna. Environ Toxicol Chem 34:931–934
Bengtsson UG, Hylander LD (2017) Increased mercury emissions from modern dental amalgams. Biometals 30(2):277–283
Jirau-Colón H, González-Parrilla L, Martinez-Jiménez J, Adam W, Jiménez-Velez B (2019) Rethinking the dental amalgam dilemma: an integrated toxicological approach. Int J Environ Res Public Health 16(6):1036
Akushevich I, Kravchenko J, Yashkin AP, Yashin AI (2018) Time trends in the prevalence of cancer and non-cancer diseases among older U.S. adults: medicare-based analysis. Exp Gerontol 110:267–276
Garza-Lombó C, Posadas Y, Quintanar L, Gonsebatt ME, Franco R (2018) Neurotoxicity linked to dysfunctional metal ion homeostatis and xenobiotic metal exposure: redox signaling and oxidative stress. Antioxid Redox Signal 28(18):1669–1703
Schofield K (2017) The metal neurotoxins: an important role in current human neural epidemics? Int J Environ Res Public Health 14(12):1511
Misra S, Boylan M, Selvam A, Spallholz JE, Björnstedt M (2015) Redox-active selenium compounds—from toxicity and cell death to cancer treatment. Nutrients 7(5):3536–3556
Vinceti M, Filippini T, Wise LA (2018) Environmental selenium and human health: an update. Curr Environ Health Rep 5(4):464–485. https://doi.org/10.1007/s40572-018-0213-0
Prasad EM, Hung SY (2020) Behavioral tests in neurotoxin-induced animal models of Parkinson’s disease. Antioxidants 9(10):1007
Yeung AWK, Georgieva MG, Atanasov AG, Tzvetkov NT (2019) Monamine oxidases (MAOs) as privileged molecular targets in neuroscience: research literature analysis. Front Mol Neurosci 12:143
Powers R, Lei S, Anandhan A, Marshall DD, Worley B, Cerny RL, Dodds ED, Huang Y, Panayiotidis MI, Pappa A, Franco R (2017) Metabolic investigations of the molecular mechanisms associated with Parkinson’s disease. Metabolities 7(2):22
Nandipati S, Litvan I (2016) Environmental exposures and Parkinson’s disease. Int J Environ Res Public Health 13(9):881
Kamel F, Tanner CM, Umbach DM, Hoppin JA, Alavanja MCR, Blair A, Comyns K et al (2007) Pesticide exposure and self-reported Parkinson’s disease in the agricultural health study. Am J Epidemiol 165(4):364–374
Prasuhn J, Davis RL, Kumar KR (2021) Targeting mitochondrial impairment in Parkinson’s disease: challenges and opportunities. Front Cell Dev Biol 5(8):615461. https://doi.org/10.3389/fcell.2020.615461
Minakaki G, Krainc D, Burbulla LF (2020) The convergence of alpha-synuclein, mitochondrial, and lysosomal pathways in vulnerability of midbrain dopaminergic neurons in Parkinson’s disease. Front Cell Dev Biol 8:580634. https://doi.org/10.3389/fcell.2020.580634
Bentea E, Verbruggen L, Massie A (2017) The proteasome inhibition model of Parkinson’s disease. J Parkinsons Dis 7(1):31–63
Ureshino RP, Erustes AG, Bassani TB, Wachilewski P, Guarache GC, Nascimento AC, Costa AJ, Smaili SS, Pereira GJDS (2019) The interplay between Ca2+ signaling pathways and neurodegeneration. Int J Mol Sci 20(23):6004. https://doi.org/10.3390/ijms20236004
Zaman V, Shields DC, Shams R, Drasites KP, Matzelle D, Haque A, Banik NL (2021) Cellular and molecular pathophysiology in the progression of Parkinson’s disease. Metab Brain Dis 36(5):815–827. https://doi.org/10.1007/s11011-021-00689-5
Henderson MX, Trojanowski JQ, Lee VM-Y (2019) α-Synuclein pathology in Parkinson’s disease and related α-synucleinopathies. Neurosci Lett 709:134316
Trist BG, Hare DJ, Double KL (2019) Oxidative stress in the aging substantia nigra and the etiology of Parkinson’s disease. Aging Cell 18(6):e13031. https://doi.org/10.1111/acel.13031
Rudyk CA, McNeill J, Prowse N, Dwyer Z, Farmer K, Litteljohn D, Caldwell W, Hayley S (2017) Age and chronicity of administration dramatically influenced the impact of low dose paraquat exposure on behavior and hypothalmic-pituitary-adrenal activity. Front Aging Neurosci 9:222
Rudyk C, Dwyer Z, McNeill J, Salmaso N, Farmer K, Prowse N, Hayley S (2019) Chronic unpredictable stress influenced the behavioral but not the neurodegenerative impact of paraquat. Neurobiol Stress 11:100179
Acknowledgements
There are no acknowledgements.
Funding
No funding was received for this article.
Author information
Authors and Affiliations
Contributions
Kevin Roe is the sole author of all contributions.
Corresponding author
Ethics declarations
Conflict of interest
The author has no potential conflicts of interest.
Ethical Approval
Not applicable.
Consent for Publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Kevin Roe—Retired.
Rights and permissions
About this article
Cite this article
Roe, K. An Alternative Explanation for Alzheimer’s Disease and Parkinson’s Disease Initiation from Specific Antibiotics, Gut Microbiota Dysbiosis and Neurotoxins. Neurochem Res 47, 517–530 (2022). https://doi.org/10.1007/s11064-021-03467-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03467-y