Abstract
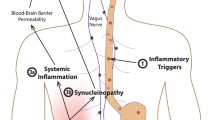
While the pathogenesis of Parkinson’s disease is not fully understood, there is increasing evidence that inflammatory responses in the brain are implicated in both disease initiation and progression. The inflammatory process in Parkinson’s disease is, however, not limited to the brain but also involves the gastrointestinal tract. High amounts of cytokines and inflammatory markers are found in the colon of Parkinson’s disease patients and there is now strong epidemiological and genetical evidence linking Parkinson’s disease to inflammatory bowel diseases. Recent findings obtained in both experimental inflammatory bowel diseases and Parkinson’s disease further support a bidirectional link between gastrointestinal inflammation and brain neurodegeneration. Altogether, these observations suggest a role for gastrointestinal inflammation in the initiation and progression of Parkinson’s disease.


Similar content being viewed by others
References
Chapelet G, Leclair-Visonneau L, Clairembault T et al (2018) Can the gut be the missing piece in uncovering PD pathogenesis? Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.11.014
Edwards LL, Quigley EM, Pfeiffer RF (1992) Gastrointestinal dysfunction in Parkinson’s disease: frequency and pathophysiology. Neurology 42:726–732
Beach TG, Adler CH, Sue LI et al (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702. https://doi.org/10.1007/s00401-010-0664-3
Gelpi E, Navarro-Otano J, Tolosa E et al (2014) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29:1010–1018. https://doi.org/10.1002/mds.25776
Wakabayashi K, Takahashi H, Takeda S et al (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221
Bialecka M, Kurzawski M, Klodowska-Duda G et al (2007) CARD15 variants in patients with sporadic Parkinson’s disease. Neurosci Res 57:473–476. https://doi.org/10.1016/j.neures.2006.11.012
Maeda S, Hsu L-C, Liu H et al (2005) Nod2 mutation in Crohn’s disease potentiates NF-kappaB activity and IL-1beta processing. Science 307:734–738. https://doi.org/10.1126/science.1103685
Umeno J, Asano K, Matsushita T et al (2011) Meta-analysis of published studies identified eight additional common susceptibility loci for Crohn’s disease and ulcerative colitis. Inflamm Bowel Dis 17:2407–2415. https://doi.org/10.1002/ibd.21651
Hui KY, Fernandez-Hernandez H, Hu J et al (2018) Functional variants in the LRRK2 gene confer shared effects on risk for Crohn’s disease and Parkinson’s disease. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aai7795
Bihari K, Lees AJ (1987) Cigarette smoking, Parkinson’s disease and ulcerative colitis. J Neurol Neurosurg Psychiatry 50:635
Fujioka S, Curry SE, Kennelly KD et al (2017) Occurrence of Crohn’s disease with Parkinson’s disease. Parkinsonism Relat Disord 37:116–117. https://doi.org/10.1016/j.parkreldis.2017.01.013
Lin J-C, Lin C-S, Hsu C-W et al (2016) Association between parkinson’s disease and inflammatory bowel disease: a Nationwide Taiwanese Retrospective Cohort Study. Inflamm Bowel Dis 22:1049–1055. https://doi.org/10.1097/MIB.0000000000000735
Peter I, Dubinsky M, Bressman S et al (2018) Anti-tumor necrosis factor therapy and incidence of parkinson disease among patients with inflammatory bowel disease. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2018.0605
Villumsen M, Aznar S, Pakkenberg B et al (2018) Inflammatory bowel disease increases the risk of Parkinson’s disease: a Danish nationwide cohort study 1977–2014. Gut. https://doi.org/10.1136/gutjnl-2017-315666
Weimers P, Halfvarson J, Sachs MC et al (2019) Inflammatory bowel disease and Parkinson’s disease: a Nationwide Swedish Cohort Study. Inflamm Bowel Dis 25:111–123. https://doi.org/10.1093/ibd/izy190
Zhu F, Li C, Gong J et al (2019) The risk of Parkinson’s disease in inflammatory bowel disease: a systematic review and meta-analysis. Dig Liver Dis 51:38–42. https://doi.org/10.1016/j.dld.2018.09.017
Marras C, Lang AE, Austin PC et al (2016) Appendectomy in mid and later life and risk of Parkinson’s disease: a population-based study. Mov Disord 31:1243–1247. https://doi.org/10.1002/mds.26670
Mendes A, Gonçalves A, Vila-Chã N et al (2015) Appendectomy may delay Parkinson’s disease onset. Mov Disord 30:1404–1407. https://doi.org/10.1002/mds.26311
Palacios N, Hughes KC, Cereda E et al (2018) Appendectomy and risk of Parkinson’s disease in two large prospective cohorts of men and women. Mov Disord 33:1492–1496. https://doi.org/10.1002/mds.109
Yilmaz R, Bayram E, Ulukan Ç et al (2017) Appendectomy history is not related to Parkinson’s disease. J Parkinsons Dis 7:347–352. https://doi.org/10.3233/JPD-171071
Svensson E, Horváth-Puhó E, Stokholm MG et al (2016) Appendectomy and risk of Parkinson’s disease: a nationwide cohort study with more than 10 years of follow-up. Mov Disord 31:1918–1922. https://doi.org/10.1002/mds.26761
Killinger BA, Madaj Z, Sikora JW et al (2018) The vermiform appendix impacts the risk of developing Parkinson’s disease. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aar5280
Altschuler SM, Escardo J, Lynn RB, Miselis RR (1993) The central organization of the vagus nerve innervating the colon of the rat. Gastroenterology 104:502–509
Gray MT, Munoz DG, Gray DA et al (2014) Alpha-synuclein in the appendiceal mucosa of neurologically intact subjects. Mov Disord 29:991–998. https://doi.org/10.1002/mds.25779
Russel MG, Dorant E, Brummer RJ et al (1997) Appendectomy and the risk of developing ulcerative colitis or Crohn’s disease: results of a large case-control study. South Limburg Inflammatory Bowel Disease Study Group. Gastroenterology 113:377–382
Devos D, Lebouvier T, Lardeux B et al (2013) Colonic inflammation in Parkinson’s disease. Neurobiol Dis 50:42–48. https://doi.org/10.1016/j.nbd.2012.09.007
Pochard C, Leclair-Visonneau L, Coron E et al (2018) Cyclooxygenase 2 is upregulated in the gastrointestinal tract in Parkinson’s disease. Mov Disord 33:493–494. https://doi.org/10.1002/mds.27237
Perez-Pardo P, Dodiya HB, Engen PA et al (2018) Role of TLR4 in the gut-brain axis in Parkinson’s disease: a translational study from men to mice. Gut. https://doi.org/10.1136/gutjnl-2018-316844
Eeckhaut V, Machiels K, Perrier C et al (2013) Butyricicoccus pullicaecorum in inflammatory bowel disease. Gut 62:1745–1752. https://doi.org/10.1136/gutjnl-2012-303611
Houser MC, Chang J, Factor SA et al (2018) Stool immune profiles evince gastrointestinal inflammation in Parkinson’s disease. Mov Disord 33:793–804. https://doi.org/10.1002/mds.27326
Schwiertz A, Spiegel J, Dillmann U et al (2018) Fecal markers of intestinal inflammation and intestinal permeability are elevated in Parkinson’s disease. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2018.02.022
Eichele DD, Kharbanda KK (2017) Dextran sodium sulfate colitis murine model: an indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J Gastroenterol 23:6016–6029. https://doi.org/10.3748/wjg.v23.i33.6016
Villarán RF, Espinosa-Oliva AM, Sarmiento M et al (2010) Ulcerative colitis exacerbates lipopolysaccharide-induced damage to the nigral dopaminergic system: potential risk factor in Parkinson’s disease. J Neurochem 114:1687–1700. https://doi.org/10.1111/j.1471-4159.2010.06879.x
Garrido-Gil P, Rodriguez-Perez AI, Dominguez-Meijide A et al (2018) Bidirectional neural interaction between central dopaminergic and gut lesions in Parkinson’s disease models. Mol Neurobiol. https://doi.org/10.1007/s12035-018-0937-8
Blandini F, Armentero M-T, Martignoni E (2008) The 6-hydroxydopamine model: news from the past. Parkinsonism Relat Disord 14(Suppl 2):S124–S129. https://doi.org/10.1016/j.parkreldis.2008.04.015
Pellegrini C, Fornai M, Colucci R et al (2016) Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflamm 13:146. https://doi.org/10.1186/s12974-016-0608-5
Zheng L-F, Wang Z-Y, Li X et al (2011) Reduced expression of choline acetyltransferase in vagal motoneurons and gastric motor dysfunction in a 6-OHDA rat model of Parkinson’s disease. Brain Res 1420:59–67. https://doi.org/10.1016/j.brainres.2011.09.006
Lema Tomé CM, Tyson T, Rey NL et al (2013) Inflammation and α-synuclein’s prion-like behavior in Parkinson’s disease—is there a link? Mol Neurobiol 47:561–574. https://doi.org/10.1007/s12035-012-8267-8
Houser MC, Tansey MG (2017) The gut-brain axis: is intestinal inflammation a silent driver of Parkinson’s disease pathogenesis? NPJ Parkinsons Dis 3:3. https://doi.org/10.1038/s41531-016-0002-0
Walter GC, Phillips RJ, Baronowsky EA, Powley TL (2009) Versatile, high-resolution anterograde labeling of vagal efferent projections with dextran amines. J Neurosci Methods 178:1–9. https://doi.org/10.1016/j.jneumeth.2008.11.003
Liu B, Fang F, Pedersen NL et al (2017) Vagotomy and Parkinson disease: a Swedish register-based matched-cohort study. Neurology 88:1996–2002. https://doi.org/10.1212/WNL.0000000000003961
Svensson E, Horváth-Puhó E, Thomsen RW et al (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529. https://doi.org/10.1002/ana.24448
Prigent A, Lionnet A, Durieu E et al (2019) Enteric alpha-synuclein expression is increased in Crohn’s disease. Acta Neuropathol 137:359–361. https://doi.org/10.1007/s00401-018-1943-7
Prigent A, Gonzales J, Durand T et al (2018) Acute inflammation down-regulates alpha-synuclein expression in enteric neurons. J Neurochem. https://doi.org/10.1111/jnc.14656
Guan Q, Zhang J (2017) Recent advances: the imbalance of cytokines in the pathogenesis of inflammatory bowel disease. Mediat Inflamm 2017:4810258. https://doi.org/10.1155/2017/4810258
Fedorova TD, Seidelin LB, Knudsen K et al (2017) Decreased intestinal acetylcholinesterase in early Parkinson disease: an 11C-donepezil PET study. Neurology 88:775–781. https://doi.org/10.1212/WNL.0000000000003633
Greenland JC, Williams-Gray CH, Barker RA (2019) The clinical heterogeneity of Parkinson’s disease and its therapeutic implications. Eur J Neurosci 49:328–338. https://doi.org/10.1111/ejn.14094
Johnson ME, Stecher B, Labrie V et al (2019) Triggers, facilitators, and aggravators: redefining Parkinson’s disease pathogenesis. Trends Neurosci 42:4–13. https://doi.org/10.1016/j.tins.2018.09.007
Zou W, Pu T, Feng W et al (2019) Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener 8:7. https://doi.org/10.1186/s40035-019-0147-y
Takagawa T, Kitani A, Fuss I et al (2018) An increase in LRRK2 suppresses autophagy and enhances Dectin-1-induced immunity in a mouse model of colitis. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aan8162
Acknowledgements
Work in our laboratory was supported by Institut de France (Fondation NRJ), France Parkinson, CECAP (Comité d’Entente et de Coordination des Associations de Parkinsoniens), ADPLA (Association des Parkinsoniens de Loire Atlantique), FFPG (Fédération française des groupements parkinsoniens) and Parkinsoniens de Vendée.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors report no disclosure relevant to the research covered in this article.
Rights and permissions
About this article
Cite this article
Rolli-Derkinderen, M., Leclair-Visonneau, L., Bourreille, A. et al. Is Parkinson’s disease a chronic low-grade inflammatory bowel disease?. J Neurol 267, 2207–2213 (2020). https://doi.org/10.1007/s00415-019-09321-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09321-0