Abstract
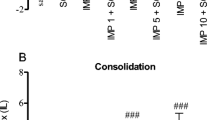
Cholinergic system dysfunction, oxidative damage, and alterations in ion pump activity have been associated with memory loss and cognitive deficits in Alzheimer’s disease. 1,3-thiazolidin-4-ones have emerged as a class of compounds with potential therapeutic effects due to their potent anticholinesterase activity. Accordingly, this study investigated the effect of the 2-(4-(methylthio)phenyl)-3-(3-(piperidin-1-yl)propyl)thiazolidin-4-one (DS12) compound on memory, cholinergic and oxidative stress parameters, ion pump activity, and serum biochemical markers in a scopolamine-induced memory deficit model. Male Wistar rats were divided into four groups: I-Control; II-Scopolamine; III-DS12 (5 mg/kg) + scopolamine; and IV-DS12 (10 mg/kg) + scopolamine. The animals from groups III and IV received DS12 diluted in canola oil and administered for 7 days by gavage. On the last day of treatment, scopolamine (1 mg/kg) was administered intraperitoneally (i.p.) 30 min after training in an inhibitory avoidance apparatus. Twenty-four hours after scopolamine administration, the animals were subjected to an inhibitory avoidance test and were thereafter euthanized. Scopolamine induced memory deficits, increased acetylcholinesterase activity and oxidative damage, and decreased Na+/K+-ATPase activity in cerebral cortex and hippocampus. Pretreatment with DS12 prevented these brain alterations. Scopolamine also induced an increase in acetylcholinesterase activity in lymphocytes and whereas butyrylcholinesterase in serum and treatment with DS12 prevented these changes. In animals treated with DS12, no changes were observed in renal and hepatic parameters when compared to the control group. In conclusion, DS12 emerged as an important multitarget compound capable of preventing neurochemical changes associated with memory deficits.
Graphic abstract








Similar content being viewed by others
References
Bai P, Wang K, Zhang P, Shi J, Cheng X, Zhang Q, Zheng C, Cheng Y, Yang J, Lu X, Sang Z (2009) Development of chalcone-O-alkylamine derivatives as multifunctional agents against Alzheimer’s disease. Eur J M Chem 183:111737. https://doi.org/10.1016/j.ejmech.2019.111737
Denver P, McClean P (2018) Distinguishing normal brain aging from the development of Alzheimer’s disease: inflammation, insulin signaling and cognition. Neural Regen Res 13:1719–1730. https://doi.org/10.4103/1673-5374.238608
Weller J, Budson A (2018) Current understanding of Alzheimer’s disease diagnosis and treatment. F1000 Rev 7:1161. https://doi.org/10.12688/f1000research.14506.1
Joe E, Ringman J (2019) Cognitive symptoms of Alzheimer’s disease: clinical management and prevention. BMJ 367:l6217. https://doi.org/10.1136/bmj.l6217
Kumar A, Nisha C, Silakari C, Sharma I, Anusha K, Gupta N, Nair P, Tripathi T, Kumar A (2016) Current and novel therapeutic molecules and targets in Alzheimer’s disease. J Formos Med Assoc 115:3–10. https://doi.org/10.1016/j.jfma.2015.04.001
Kumar D, Gupta SK, Ganeshpurkar A, Gutti G, Krishnamurthy S, Modi G, Singh SK (2018) Development of Piperazinediones as dual inhibitor for treatment of Alzheimer’s disease. Eur J Med Chem 150:87–101. https://doi.org/10.1016/j.ejmech.2018.02.078
Xie S, Wang XB, Li J, Yang L, Kong L (2013) Design, synthesis and evaluation of novel tacrine-coumarin hybrids as multifunctional cholinesterase inhibitors against Alzheimer’s disease. Eur J Med Chem 64:540–553. https://doi.org/10.1016/j.ejmech.2013.03.051
Tang KS (2019) The cellular and molecular processes associated with scopolamine-induced memory deficit: a model of Alzheimer’s biomarkers. Life Sci 223:116695. https://doi.org/10.1016/j.lfs.2019.116695
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol 53:648–661. https://doi.org/10.1007/s12035-014-9053-6
Ishola I, Akinyede A, Eloke JE, Chaturvedi JP, Narender T (2019) Diastereomeric mixture of calophyllic and isocalophyllic acid ameliorates scopolamine-induced memory impairment in mice: involvement of antioxidant defense and cholinergic systems. Neurotox Res 37:58–66. https://doi.org/10.1007/s12640-019-00117-8
Malviyaa M, Kumar YCS, Mythri RB, Venkateshappa C, Subhash MN, Rangappa KS (2009) Muscarinic receptor 1 agonist activity of novel N-aryl carboxamide substituted 3-morpholino arecoline derivatives in Alzheimer’s presenile dementia models. Bioorg Med Chem 17:5526–5534. https://doi.org/10.1016/j.bmc.2009.06.032
Sadashiva CT, Chandra J, Kavitha CV, Thimmegowda A, Subhash MN, Rangappa KS (2009) Synthesis and pharmacological evaluation of novel N-alkyl/aryl substituted thiazolidinone arecoline analogues as muscarinic receptor 1 agonist in Alzheimer’s dementia models. Eur J Med Chem 44:4848–4854. https://doi.org/10.1016/j.ejmech.2009.07.026
Silva DS, Soares MSP, Martini F, Pesarico AP, Mattos B, Souza AA, Silva CEH, Scaini JLR, Machado KS, Nogueira CW, Spanevello RM, Cunico W (2020) In vitro effects of 2-{4-[methylthio(methylsulfonyl)]phenyl}-3-substitutedthiazolidin-4-ones on the acetylcholinesterase activity in rat brain and lymphocytes: isoform selectivity, kinetic analysis, and molecular docking. Neurochem Res 45:241–253. https://doi.org/10.1007/s11064-019-02929-8
Berwaldt GA, Gouvêa DP, Silva DS, Neves AM, Soares MSP, Azambuja JH, Siqueira GM, Spanevello RM, Cunico W (2019) Synthesis and biological evaluation of benzothiazin-4-ones: a possible new class of acetylcholinesterase inhibitors. J Enzyme Inhib Med Chem 34:197–203. https://doi.org/10.1080/14756366.2018.1543286
Silva D, Silva C, Soares M, Azambuja J, Carvalho T, Zimmer G, Frizzo C, Braganhol E, Spanevello R, Cunico W (2016) Thiazolidin-4-ones from 4-(methylthio)benzaldehyde and 4-(methylsulfonyl)benzaldehyde: synthesis, antiglioma activity and cytotoxicity. Eur J Med Chem 124:574–582. https://doi.org/10.1016/j.ejmech.2016.08.057
Ali EH, Arafa NM (2011) Comparative protective action of curcumin, memantine and diclofenac against scopolamine-induced memory dysfunction. Fitoterapia 82:601–608. https://doi.org/10.1016/j.fitote.2011.01.016
Marisco P, Carvalho F, Rosa M, Girardi B, Gutierres J, Jaques J, Salla A, Pimentel V, Schetinger M, Leal D, Mello C, Rubin M (2013) Piracetam prevents scopolamine-induced memory impairment and decrease of NTPDase, 5-nucleotidase and adenosine deaminase activities. Neurochem Res 38:1704–1714. https://doi.org/10.1007/s11064-013-1072-6
Da Silveira EF, Azambuja JH, Carvalho TR, Kunzler A, Silva DS, Teixeira FC, Rodrigues R, Beira FT, Alvez RCSA, Spanevello RM, Cunico W, Stefanello FM, Horn AP, Braganhol E (2017) Synthetic 2-aryl-3-((piperidin-1-yl)ethyl)thiazolidin-4-ones exhibit selective in vitro antitumoral activity and inhibit cancer cell growth in a preclinical model of glioblastoma multiforme. Chem Biol Interact 266:1–9. https://doi.org/10.1016/j.cbi.2017.02.001
Da Silveira EF, Ferreira LM, Gehrcke M, Cruz L, Pedra NS, Ramos PT, Bona NP, Soares MSP, Rodrigues R, Spanevello RM, Cunico W, Stefanello FM, Azambuja JH, Horn AP, Braganhol E (2019) 2-(2-Methoxyphenyl)-3-((piperidin-1-yl)ethyl) thiazolidin-4-one-loaded polymeric nanocapsules. In vitro antiglioma activity and in vivo toxicity evaluation. Cell Mol Neurobiol 39:783–797. https://doi.org/10.1007/s10571-019-00678-4
Ellman G, Courtney K, Andres V, Featherstone R (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 17:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Wyse A, Streck E, Barros S, Brusque A, Zugno A, Wajner M (2007) Methylmalonate administration decreases Na+, K+-ATPase activity in cerebral cortex of rats. NeuroReport 11:2331–2334. https://doi.org/10.1097/00001756-200007140-00052
Chan K, Delfert D, Junger KD (1986) A direct colorimetric assay for Ca2+–ATPase activity. Anal Biochem 157:1375–1378. https://doi.org/10.1016/0003-2697(86)90640-8
Ali SF, LeBel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13:637–648
Stuehr DJ, Nathan CF (1989) Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med 169:1543–1555. https://doi.org/10.1084/jem.169.5.1543
Aksenov MY, Markesbery WR (2011) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145. https://doi.org/10.1016/S0304-3940(01)01636-6
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-H
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/S0076-6879(84)05016-3
Böyum A (1968) Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl 97:77–89
Jaques JAS, Rezer JF, Ruchel JB, Gutierres J, Bairros AV, Farias IL, Luz S, Bertoncheli C, Schetinger M, Morsch V, Leal D (2011) A method for isolation of rat lymphocyte-rich mononuclear cells from lung tissue useful for determination of nucleoside triphosphate diphosphohydrolase activity. Anal Biochem 410:34–39. https://doi.org/10.1016/j.ab.2010.10.039
Fitzgerald B, Costa L (1993) Modulation of muscarinic receptors an acetylcholinesterase activity in lymphocytes and brain areas following repeated organophosphate exposure in rats. Fund Appl Toxicol 20:210–216. https://doi.org/10.1006/faat.1993.1028
Lowry O, Rosebrough N, Farr A, Randall R (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Bradford M (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Barkur R, Bairy L (2015) Evaluation of passive avoidance learning and spatial memory in rats exposed to low levels of lead during specific periods of early brain development. Int J Occup Med Environ Health 28:533–544
Auti ST, Kulkarni YA (2019) Neuroprotective effect of cardamom oil against aluminum induced neurotoxicity in rats. Front Neurol 10:399. https://doi.org/10.3389/fneur.2019.00399
Teixeira F, Gutierres J, Soares M, Mattos B, Spohr L, Couto C, Bona N, Assmann C, Morsch V, Cruz I, Stefanello F, Spanevello RM (2020) Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacology 237:811–823. https://doi.org/10.1007/s00213-019-05419-5
Wang D, Hu M, Li X, Zhang D, Chen C, Fu J, Shao S, Shi G, Zhou Y, Wu S, Zhang T (2019) Design, synthesis, and evaluation of isoflavone analogs as multifunctional agents for the treatment of Alzheimer’s disease. Eur J Med Chem 168:207–220. https://doi.org/10.1016/j.ejmech.2019.02.053
Gutierres JM, Carvalho FB, Schetinger MRC, Agostinho P, Marisco PC, Vieira JM, Rosa MM, Bohnert C, Rubin MA, Morsch VM, Spanevello R, Mazzanti CM (2014) Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int J Dev Neurosci 33:88–97. https://doi.org/10.1016/j.ijdevneu.2013.12.006
Preston AR, Eichenbaum H (2013) Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23:764–773. https://doi.org/10.1016/j.cub.2013.05.041
Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T (2010) Abnormal cortical networks in mild cognitive impairment and Alzheimer’s disease. PLoS Comput Biol 18:e1001006. https://doi.org/10.1371/journal.pcbi.1001006
Bartorelli L, Giraldi C, Saccardo M, Cammarata S, Bottini G, Fasanaro AM, Trequattrini A (2005) Effects of switching from an AChE inhibitor to a dual AChE-BuChE inhibitor in patients with Alzheimer’s disease. Curr Med Res Opin 21:1809–1817. https://doi.org/10.1185/030079905X65655
Sun K, Bai Y, Zhao R, Guo Z, Su X, Li P, Yang P (2019) Neuroprotective effects of matrine on scopolamine-induced amnesia via inhibition of AChE/BuChE and oxidative stress. Metab Brain Dis 34:173–181. https://doi.org/10.1007/s11011-018-0335-y
Holm T, Isaksen T, Glerup S, Heuck A, Bøttger P, Füchtbauer E, Nedergaard E, Nyengaard J, Andreasen M, Nissen P, Lykke-Hartmann K (2016) Cognitive deficits caused by a disease-mutation in the α3 Na+/K+-ATPase isoform. Sci Rep 23:31972. https://doi.org/10.1038/srep31972
Zhang L, Suna Y, Panb S, Lic J, Qud J, Lia Y, Wang Y, Gao Z (2013) Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fund Clin Pharmacol 27:96–103. https://doi.org/10.1111/fcp.12000
Luchese C, Vogt A, Pinz M, Reis A, Gomes C, Alves D, Wilhelm E (2020) Amnesia-ameliorative effect of a quinoline derivative through regulation of oxidative/cholinergic systems and Na+/K+-ATPase activity in mice. Metab Brain Dis 35:589–600. https://doi.org/10.1007/s11011-020-00535-0
Silva F, Pinz M, Oliveira R, Rodrigues K, Ianiski F, Bassaco M, Silveira C, Jesse C, Roman S, Wilhelm E, Luchese C (2017) Organosulfur compound protects against memory decline induced by scopolamine through modulation of oxidative stress and Na+/K+ ATPase activity in mice. Metab Brain Dis 32:1819–1828. https://doi.org/10.1007/s11011-017-0067-4
Fan Y, Hu J, Li J, Yang Z, Xin X, Wang J, Ding J, Geng M (2005) Effect of acidic oligosaccharide sugar chain on scopolamine-induced memory impairment in rats and its related mechanisms. Neurosci Lett 374:222–226. https://doi.org/10.1016/j.neulet.2004.10.063
Ajami M, Eghtesadia S, Habibeyb R, Razaz JM, Peyrovid H, Zarrindast M, Pazoki-Toroudib H (2012) Effect of short and long-term treatment with omega-3 fatty acids on scopolamine-induced amnesia. Iran J Pharm Res 11:533–540
Hancianu M, Cioanca O, Mihasan M, Hritcu L (2013) Neuroprotective effects of inhaled lavender oil on scopolamine-induced dementia via anti-oxidative activities in rats. Phytomedicine 20:446–452. https://doi.org/10.1016/j.phymed.2012.12.005
Qu Z, Zhang J, Yang H, Gao J, Chen H, Liu C, Gao W (2017) Prunella vulgaris L., an edible and medicinal plant, attenuates scopolamine-induced memory impairment in rats. Agric Food Chem 65:291–300. https://doi.org/10.1021/acs.jafc.6b04597
Agostinho P, Cunha R, Oliveira C (2010) Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer’s disease. Curr Pharm Des 16:2766–2778. https://doi.org/10.2174/138161210793176572
Acknowledgments
The authors tank veterinarian doctor Anelize de Oliveira Campello Felix for helping with animal experimentation.
Funding
This research was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS—PRONEM processo: 16/2551–0000 2452). This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior-Brasil (CAPES)—Finance code 001. W. C. and R. M. S. are recipients of the CNPq fellowship.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
All animal experimental protocols were approved by the Committee of Ethics and Animal Experimentation of the Federal University of Pelotas, RS, Brazil (protocol number: CEEA 46528-2018).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
da Silva, D.S., Soares, M.S.P., Teixeira, F.C. et al. Multitarget Effect of 2-(4-(Methylthio)phenyl)-3-(3-(piperidin-1-yl)propyl)thiazolidin-4-one in a Scopolamine-Induced Amnesic Rat Model. Neurochem Res 46, 1554–1566 (2021). https://doi.org/10.1007/s11064-021-03295-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03295-0