Abstract
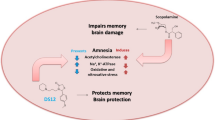
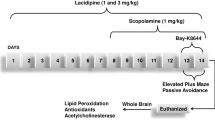
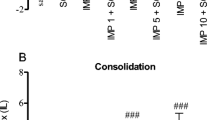
This study investigated the effects of inosine on memory acquisition and consolidation, cholinesterases activities, redox status and Na+, K+-ATPase activity in a rat model of scopolamine-induced cognitive impairment. Adult male rats were divided into four groups: control (saline), scopolamine (1 mg/kg), scopolamine plus inosine (50 mg/kg), and scopolamine plus inosine (100 mg/kg). Inosine was pre-administered for 7 days, intraperitoneally. On day 8, scopolamine was administered pre (memory acquisition protocol) or post training (memory consolidation protocol) on inhibitory avoidance tasks. The animals were subjected to the step-down inhibitory avoidance task 24 hours after the training. Scopolamine induced impairment in the acquisition and consolidation phases; however, inosine was able to prevent only the impairment in memory consolidation. Also, scopolamine increased the activity of acetylcholinesterase and reduced the activity of Na+, K+-ATPase and the treatment with inosine protected against these alterations in consolidation protocol. In the animals treated with scopolamine, inosine improved the redox status by reducing the levels of reactive oxygen species and thiobarbituric acid reactive substances and restoring the activity of the antioxidant enzymes, superoxide dismutase and catalase. Our findings suggest that inosine may offer protection against scopolamine-induced memory consolidation impairment by modulating brain redox status, cholinergic signaling and ion pump activity. This compound may provide an interesting approach in pharmacotherapy and as a prophylactic against neurodegenerative mechanisms involved in Alzheimer’s disease.
Graphic Abstract










Similar content being viewed by others
Data Availability
Not applicable.
Code Availability
Not applicable.
References
Abel T, Lattal K (2001) Molecular mechanisms of memory acquisition, consolidation and retrieval. Curr Opin Neurobiol 11:180–187. https://doi.org/10.1016/s0959-4388(00)00194-x
Dudai Y, Karni A, Born J (2015) The consolidation and transformation of memory. Neuron 88:20–32. https://doi.org/10.1016/j.neuron.2015.09.004
Lane CA, Hardy J, Schott JM (2018) Alzheimer’s disease. Eur J Neurol 25:59–70. https://doi.org/10.1111/ene.13439
Singh RK (2020) Recent trends in the management of Alzheimer’s disease: current therapeutic options and drug repurposing approaches. Curr Neuropharmacol 18:868–882. https://doi.org/10.2174/1570159X18666200128121920
Haider S, Tabassum S, Perveen T (2016) Scopolamine-induced greater alterations in neurochemical profile and increased oxidative stress demonstrated a better model of dementia: a comparative study. Brain Res Bull 127:234–247. https://doi.org/10.1016/j.brainresbull.2016.10.002
Teixeira FC, Gutierres JM, Soares MSPS, Mattos B, Spohr L, Couto CAT, Bona N, Assmann CE, Cruz IBM, Morsch V, Stefanello FM, Spanevello RS (2020) Inosine protects against impairment of memory induced by experimental model of Alzheimer disease: a nucleoside with multitarget brain actions. Psychopharmacology 237:811–823. https://doi.org/10.1007/s00213-019-05419-5
Stanciu GD, Luca A, Rusu RN, Bild V, Chiriac SIB, Solcan C, Bild W, Ababei DC (2019) Alzheimer’s disease pharmacotherapy in relation to cholinergic system involvement. Biomolecules 10:40. https://doi.org/10.3390/biom10010040
Tönnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzheimer Dis 57:1105–1121. https://doi.org/10.3233/JAD-161088
Amato A, Terzo S, Mulè F (2019) Natural compounds as beneficial antioxidant agents in neurodegenerative disorders: a focus on Alzheimer’s disease. Antioxidants 8:608. https://doi.org/10.3390/antiox8120608
Moseley A, Williams MT, Schaefer TL, Bohanan C, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB (2007) Deficiency in Na, K-ATPase α isoform genes alter spatial learning, motor activity, and anxiety in mice. J Neurosci 27:616–626. https://doi.org/10.1523/JNEUROSCI.4464-06.2007
Pacheco SM, Soares MSP, Gutierres JM, Gerzson MFB, Carvalho FB, Azambuja JH, Schetinger MRC, Stefanello FM, Spanevello RM (2018) Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J Nutr Biochem 56:193–204. https://doi.org/10.1016/j.jnutbio.2018.02.014
Ianiski FR, Alves C, Ferreira CF, Rech VC, Savegnago L, Wilhelm EA, Luchese C (2016) Meloxicam-loaded nanocapsules as an alternative to improve memory decline in an Alzheimer’s disease model in mice: involvement of Na+, K+-ATPase. Metab Brain Dis 31:793–802. https://doi.org/10.1007/s11011-016-9812-3
Gutierres JM, Carvalho FB, Schetinger MRC, Agostinho P, Marisco P, Vieira J, Rosa M, Bohnert C, Rubin M, Morsch VM, Spanevello RM, Mazzanti CM (2014) Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int J Dev Neurosci 33:88–97. https://doi.org/10.1016/j.ijdevneu.2013.12.006
Hattori N, Kitagawa K, Higashida T, Yagyu K, Shimohama S, Wataya T, Perry G, Smith MA, Inagaki C (1998) Cl−-ATPase and Na+/K+-ATPase activities in Alzheimer’s disease brains. Neurosci Lett 254:141–144. https://doi.org/10.1016/s0304-3940(98)00654-5
Ohnishi T, Yanazawa M, Sasahara T, Kitamura Y, Hiroaki H, Fukazawa Y, Kii I, Nishiyama T, Kakita A, Takeda H, Takeuchi A, Arai Y, Ito A, Komura H, Hirao H, Satomura K, Inoue M, Muramatsu S, Matsui K, Tada M, Sato M, Saijo E, Shigemitsu Y, Sakai S, Umetsu Y, Goda N, Takino N, Takahashi H, Hagiwara M, Sawasaki T, Iwasaki G, Nakamura Y, Nabeshima Y, Teplow D, Hoshi M (2015) Na, K-ATPase α3 is a death target of Alzheimer patient amyloid-β assembly. Proc Natl Acad Sci USA 112:4465–4474. https://doi.org/10.1073/pnas.1421182112
Kaster M, Budni J, Gazal M, Cunha M, Santos A, Rodrigues A (2013) The antidepressant-like effect of inosine in the FST is associated with both adenosine A1 and A2A receptors. Purinergic Signal 9:481–486. https://doi.org/10.1007/s11302-013-9361-8
Cipriani S, Bakshi R, Schwarzschild M (2014) Protection by inosine in a cellular model of Parkinson’s disease. Neuroscience 274:242–249. https://doi.org/10.1016/j.neuroscience.2014.05.038
Ruhal P, Dhingra D (2018) Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacology 26:1317–1329. https://doi.org/10.1007/s10787-018-0476-y
Haskó G, Sitkovsky MV, Szabó C (2004) Immunomodulatory and neuroprotective effects of inosine. Trends Pharmacol Sci 25:152–157. https://doi.org/10.1016/j.tips.2004.01.006
Shen H, Chen G, Harvey B, Bickford P, Wang Y (2005) Inosine reduces ischemic brain injury in rats. Stroke 36:654–659. https://doi.org/10.1161/01.STR.0000155747.15679.04
Schwarzschild MA et al (2014) Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol 71:141–150. https://doi.org/10.1001/jamaneurol.2013.5528
Doyle C, Cristofaro V, Sullivan MP, Adam R (2018) Inosine—a multifunctional treatment for complications of neurologic injury. Cell Physiol Biochem 49:2293–2303. https://doi.org/10.1159/000493831
Gutierres GM, Carvalho FB, Schetinger MRC, Rodrigues M, Schmatz R, Pimentel V, Vieira J, Rosa M, Marisco M, Ribeiro D, Leal C, Rubin M, Mazzanti CM, Spanevello R (2012) Protective effects of anthocyanins on the ectonucleotidase activity in the impairment of memory induced by scopolamine in adult rats. Life Sci 91:1221–1228. https://doi.org/10.1016/j.lfs.2012.09.013
Wong-Guerra M, Jiménez-Martin J, Fonseca-Fonseca LA, Ramírez-Sánchez J, Montano-Peguero Y, Rocha JB, Avila F, Assis A, Souza DO, Pardo-Andreu GL, Del Valle RM, Lopez GA, Martínez OV, García NM, Mondelo-Rodríguez A, Padrón-Yaquis A, Nuñez-Figueredo Y (2019) JM-20 protects memory acquisition and consolidation on scopolamine model of cognitive impairment. Neurol Res 41:385–398. https://doi.org/10.1080/01616412.2019.1573285
Dachir S, Shabashov D, Trembovler V, Alexandrovich AG, Benowitz L, Shohami E (2014) Inosine improves functional recovery after experimental traumatic brain injury. Brain Res 1555:78–88. https://doi.org/10.1016/j.brainres.2014.01.044
Ellman GL, Courtney KD, Andres V, Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Lowry OH, Rosebrough NG, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ali SF, Lebel CP, Bondy SC (1992) Reactive oxygen species formation as a biomarker of methylmercury and trimethyltin neurotoxicity. Neurotoxicology 13:637–648
Huang W, Lin S, Wang C, Tsai C, Tseng H, Chen C, Lu P, Chen P, Qian L, Hong J, Lin C (2009) Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 128:275–286. https://doi.org/10.1111/j.1365-2567.2008.02959.x
Aksenov MY, Markesbery WR (2001) Changes in thiol content and expression of glutathione redox system genes in the hippocampus and cerebellum in Alzheimer’s disease. Neurosci Lett 302:141–145. https://doi.org/10.1016/s0304-3940(01)01636-6
Esterbauer H, Cheeseman KH (1990) Determination of aldehydic lipid peroxidation products: malonaldehyde and 4-hydroxynonenal. Methods Enzymol 186:407–421. https://doi.org/10.1016/0076-6879(90)86134-h
Misra HP, Fridovich I (1972) The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126. https://doi.org/10.1016/s0076-6879(84)05016-3
Carvalho FB, Mello CF, Marisco P, Tonello R, Girardi B, Ferreira J, Oliveira M, Rubin M (2012) Spermidine decreases Na+, K+-ATPase activity through NMDA receptor and protein kinase G activation in the hippocampus of rats. Eur J Pharmacol 684:79–86. https://doi.org/10.1016/j.ejphar.2012.03.046
Gilles C, Ertlé S (2000) Pharmacological models of Alzheimer’s disease research. Dialogues Clin Neurosci 3:247–255. https://doi.org/10.31887/DCNS.2000.2.3/cgilles
Klinkenberg I, Blokland A (2010) The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci Biobehav Rev 34:1307–1350. https://doi.org/10.1016/j.neubiorev.2010.04.001
Tang S (2019) The cellular and molecular processes associated with scopolamine-induced memory deficit: a model of Alzheimer’s biomarkers. Life Sci 233:116695. https://doi.org/10.1016/j.lfs.2019.116695
Da Silva F, Pinz M, Oliveira RL, Rodrigues K, Ianiski F, Bassaco M, Silveira C, Jesse CR, Roman SS, Wilhelm E, Luchese C (2017) Organosulfur compound protects against memory decline induced by scopolamine through modulation of oxidative stress and Na+/K+ ATPase activity in mice. Metab Brain Dis 32:1819–1828. https://doi.org/10.1007/s11011-017-0067-4
Ozawa T, Yamada K, Ichitani Y (2019) d-Cycloserine reverses scopolamine-induced object and place memory deficits in a spontaneous recognition paradigm in rats. Pharmacol Biochem Behav 187:172798. https://doi.org/10.1016/j.pbb.2019.172798
Moulin C, James N, Jones R, Freeman J (2004) Deficient acquisition and consolidation: intertrial free recall performance in Alzheimer’s disease and mild cognitive impairment. J Clin Exp Neuropsychol 26:1–10. https://doi.org/10.1076/jcen.26.1.1.23940
Deutsch JA (1971) The cholinergic synapse and the site of memory. Science 174:788–794. https://doi.org/10.1126/science.174.4011.788
Schneider L (2001) Treatment of Alzheimer’s disease with cholinesterase inhibitors. Clin Geriatr Med 17:337–339. https://doi.org/10.1016/s0749-0690(05)70072-0
Haake A, Nguyen K, Friedman L, Chakkamparamil B, Grossberg GT (2020) An update on the utility and safety of cholinesterase inhibitors for the treatment of Alzheimer’s disease. Expert Opin Drug Saf 19:147–157. https://doi.org/10.1080/14740338.2020.1721456
Rao A, Gumpeny S, Das U (2007) Elevated butyrylcholinesterase and acetylcholinesterase may predict the development of type 2 diabetes mellitus and Alzheimer’s disease. Med Hypotheses 69:1272–1276. https://doi.org/10.1016/j.mehy.2007.03.032
Mushtaq G, Greig N, Khan J, Mohammad K (2014) Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s diseases and type 2 diabetes mellitus. CNS Neurol Dis Drug Targets 13:1432–1439. https://doi.org/10.2174/1871527313666141023141545
Ha Z, Mathew S, Yeong K (2020) Butyrylcholinesterase: a multifaceted pharmacological target and tool. Curr Protein Pept Sci 21:99–109. https://doi.org/10.2174/1389203720666191107094949
Ishola I, Akinyede A, Eloke J, Chatuverdi J, Narender T (2019) Diastereomeric mixture of calophyllic and isocalophyllic acid ameliorates scopolamine-induced memory impairment in mice: involvement of antioxidant defense and cholinergic systems. Neurotox Res 37:58–66. https://doi.org/10.1007/s12640-019-00117-8
Qu Z, Zhang J, Yang H, Gao J, Chen H, Liu C, Gao W (2017) Prunella vulgaris L. an edible and medicinal plant, attenuates scopolamine-induced memory impairment in rats. J Agric Food Chem 65:291–300. https://doi.org/10.1021/acs.jafc.6b04597
Venkatesan R, Subedi L, Yeo EJ, Kim SJ (2016) Lactucopicrin ameliorates oxidative stress mediated by scopolamine-induced neurotoxicity through activation of the NRF2 pathway. Neurochem Int 99:133–146. https://doi.org/10.1016/j.neuint.2016.06.010
Zhang LN, Sun YJ, Pan S, Li JX, Qu YE, Li Y, Wang YL, Gao ZB (2013) Na+-K+-ATPase, a potent neuroprotective modulator against Alzheimer disease. Fundam Clin Pharmacol 27:96–103. https://doi.org/10.1111/fcp.12000
Arnaiz G, Ordieres M (2014) Brain Na+, K+-ATPase activity in aging and disease. Int J Biomed Sci 10:85–112
Pagnussat N, Almeida AS, Marques DM, Nunes F, Chenet GC, Botton PHS, Mioranza S, Loss CM, Cunha RA, Porciúncula LO (2015) Adenosine A2A receptors are necessary and sufficient to trigger memory impairment in adult mice. Br J Pharmacol 172:3831–3845. https://doi.org/10.1111/bph.13180
Kim D, Ryu J (2008) Activation of adenosine A2A receptor impairs memory acquisition but not consolidation or retrieval phases. Biomol Ther 16:320–327. https://doi.org/10.4062/biomolther.2008.16.4.320
Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Bianchin M, Izquerdo I, Medina J (1997) Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA 94:7041–7046. https://doi.org/10.1073/pnas.94.13.7041
Acknowledgements
We thank the Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo à Pesquisa do Rio Grande do Sul. This study was also financed in part by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brazil (CAPES—Finance code 001). We would like to thank Editage (www.editage.com) for English language editing.
Funding
This research was supported by Fundação de Amparo à Pesquisa do Rio Grande do Sul (FAPERGS Grant Number—19/2551-0001712-0).
Author information
Authors and Affiliations
Contributions
Conceptualization (FCT, JMG, RMS), data curation (BSM, JEM, JC, LS, KPL), formal analysis (FCT, MSPS), funding acquisition (FMS, RMS), animals procedures (FCT, AOCF, BSM, JEM, JC), biochemical analysis (FCT, LS, KPL, MSPS), manuscript elaboration and review (FCT, FC, JMG, RMS).
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that there are no conflicts of interest.
Ethical Approval
All animal experimental protocols were approved by the Committee of Ethics and Animal Experimentation of the Federal University of Pelotas, RS, Brazil (Protocol Number: CEEA 4808- 2017).
Informed Consent
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Teixeira, F.C., de Mattos, B.S., Mello, J.E. et al. Protective Effects of Inosine on Memory Consolidation in a Rat Model of Scopolamine-Induced Cognitive Impairment: Involvement of Cholinergic Signaling, Redox Status, and Ion Pump Activities. Neurochem Res 47, 446–460 (2022). https://doi.org/10.1007/s11064-021-03460-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-021-03460-5