Abstract
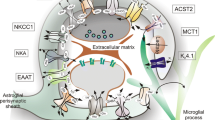
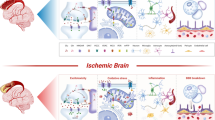
Cerebral ischemia leads to reactive astrogliosis and glial scar formation. Glial scarring can impede functional restoration during the recovery phase of stroke. Salidroside has been shown to have neuroprotective effects after ischemic stroke, but its impact on long-term neurological recovery, especially whether it regulates reactive astrogliosis and glial scar formation, is unclear. In this study, male adult C57/BL6 mice were subjected to transient cerebral ischemia injury followed by intravenous salidroside treatment. Primary astrocytes were treated with lipopolysaccharide (LPS) or conditioned medium from cultured primary neurons subjected to oxygen-glucose deprivation (CM-OGD). Salidroside significantly improved long-term functional outcomes following ischemic stroke in the rotarod and corner tests. It also reduced brain glial scar volume and decreased expression of the glial scar marker, glial fibrillary acidic protein (GFAP) and inhibited astrocyte proliferation. In primary astrocyte cultures, salidroside protected astrocytes from CM-OGD injury-induced reactive astroglial proliferation, increasing the percentage of cells in G0/G1 phase and reducing the S populations. The inhibitory effect of salidroside on the cell cycle was related to downregulation of cyclin D1 and cyclin-dependent kinase 4 (CDK4) mRNA expression and increased p27Kip1 mRNA expression. Similar results were found in the LPS-stimulated injury model in astroglial cultures. Western blot analysis demonstrated that salidroside attenuated the CM-OGD-induced upregulation of phosphorylated Akt and glycogen synthase kinase 3β (GSK-3β). Taken together, these results suggested that salidroside can inhibit reactive astrocyte proliferation, ameliorate glial scar formation and improve long-term recovery, probably through its effects on the Akt/GSK-3β pathway.









Similar content being viewed by others
Abbreviations
- BrdU:
-
5-bromo-2′-deoxyuridine
- CDK4:
-
Cyclin-dependent kinase 4
- CM-OGD:
-
Conditioned medium suffering from oxygen-glucose deprivation
- CNS:
-
Central nervous system
- DMEM:
-
Dulbecco’s modified Eagle’s medium
- FBS:
-
Fetal bovine serum
- GFAP:
-
Glial fibrillary acidic protein
- GSK-3β:
-
Glycogen synthase kinase 3β
- LPS:
-
Lipopolysaccharide
- MAP2:
-
Microtubule-associated protein 2
- MCAO:
-
Middle cerebral artery occlusion
- OGD:
-
Oxygen-glucose deprivation
- PBS:
-
Phosphate buffered saline
- SD:
-
Sprague-Dawley
- tPA:
-
Tissue plasminogen activator.
References
Feigin VL, Krishnamurthi RV, Parmar P et al (2015) Update on the global burden of ischemic and hemorrhagic stroke in 1990-2013: the GBD 2013 study. Neuroepidemiology 45:161–176
Avan A, Digaleh H, Di Napoli M et al (2019) Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the global burden of disease study 2017. BMC Med 17:191
Damani R (2018) A brief history of acute stroke care. Aging (Albany NY) 10:1797–1798
Chapman SN, Mehndiratta P, Johansen MC et al (2014) Current perspectives on the use of intravenous recombinant tissue plasminogen activator (tPA) for treatment of acute ischemic stroke. Vasc Health Risk Manag 10:75–87
Dewar B, Shamy M (2020) tPA for acute ischemic stroke and its controversies: a review. Neurohospitalist 10:5–10
Nedergaard M, Ransom B, Goldman SA (2003) New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci 26:523–530
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Wang C, Jie C, Dai X (2014) Possible roles of astrocytes in estrogen neuroprotection during cerebral ischemia. Rev Neurosci 25:255–268
Dias DO, Goritz C (2018) Fibrotic scarring following lesions to the central nervous system. Matrix Biol 68-69:561–570
Cregg JM, DePaul MA, Filous AR et al (2014) Functional regeneration beyond the glial scar. Exp Neurol 253:197–207
Sun L, Zhang Y, Liu E et al (2019) The roles of astrocyte in the brain pathologies following ischemic stroke. Brain Inj 33:712–716
Chiang HM, Chen HC, Wu CS et al (2015) Rhodiola plants: chemistry and biological activity. J Food Drug Anal 23:359–369
Pu WL, Zhang MY, Bai RY et al (2020) Anti-inflammatory effects of Rhodiola rosea L.: a review. Biomed Pharmacother 121:109552
Han J, Xiao Q, Lin YH et al (2015) Neuroprotective effects of salidroside on focal cerebral ischemia/reperfusion injury involve the nuclear erythroid 2-related factor 2 pathway. Neural Regen Res 10:1989–1996
Chen T, Ma Z, Zhu L et al (2016) Suppressing receptor-interacting protein 140: a new sight for Salidroside to treat cerebral ischemia. Mol Neurobiol 53:6240–6250
Liu X, Wen S, Yan F et al (2018) Salidroside provides neuroprotection by modulating microglial polarization after cerebral ischemia. J Neuroinflammation 15:39
Zhang X, Du Q, Yang Y et al (2018) Salidroside alleviates ischemic brain injury in mice with ischemic stroke through regulating BDNK mediated PI3K/Akt pathway. Biochem Pharmacol 156:99–108
Yu S, Xu H, Chi X et al (2018) 2-(4-Methoxyphenyl)ethyl-2-Acetamido-2-deoxy-beta-d-pyranoside (A Salidroside analog) confers neuroprotection with a wide therapeutic window by regulating local glucose metabolism in a rat model of cerebral ischemic injury. Neuroscience 391:60–72
Zuo W, Yan F, Zhang B et al (2018) Salidroside improves brain ischemic injury by activating PI3K/Akt pathway and reduces complications induced by delayed tPA treatment. Eur J Pharmacol 830:128–138
Wei Y, Hong H, Zhang X et al (2017) Salidroside inhibits inflammation through PI3K/Akt/HIF signaling after focal cerebral ischemia in rats. Inflammation 40:1297–1309
Lai W, Xie X, Zhang X et al (2018) Inhibition of complement drives increase in early growth response proteins and neuroprotection mediated by Salidroside after cerebral ischemia. Inflammation 41:449–463
Shi TY, Feng SF, Xing JH et al (2012) Neuroprotective effects of Salidroside and its analogue tyrosol galactoside against focal cerebral ischemia in vivo and H2O2-induced neurotoxicity in vitro. Neurotox Res 21:358–367
Hu X, Li P, Guo Y et al (2012) Microglia/macrophage polarization dynamics reveal novel mechanism of injury expansion after focal cerebral ischemia. Stroke 43:3063–3070
Zhang L, Schallert T, Zhang ZG et al (2002) A test for detecting long-term sensorimotor dysfunction in the mouse after focal cerebral ischemia. J Neurosci Methods 117:207–214
Liu X, Liu J, Zhao S et al (2016) Interleukin-4 is essential for microglia/macrophage M2 polarization and long-term recovery after cerebral ischemia. Stroke 47:498–504
Ciccarelli R, D’Alimonte I, Ballerini P et al (2007) Molecular signalling mediating the protective effect of A1 adenosine and mGlu3 metabotropic glutamate receptor activation against apoptosis by oxygen/glucose deprivation in cultured astrocytes. Mol Pharmacol 71:1369–1380
Huang XQ, Zhang XY, Wang XR et al (2012) Transforming growth factor beta1-induced astrocyte migration is mediated in part by activating 5-lipoxygenase and cysteinyl leukotriene receptor 1. J Neuroinflammation 9:145
Chen Y, Zhang J, Deng M (2015) Furin mediates brain-derived neurotrophic factor upregulation in cultured rat astrocytes exposed to oxygen-glucose deprivation. J Neurosci Res 93:189–194
Liddelow SA, Barres BA (2017) Reactive astrocytes: production, function, and therapeutic potential. Immunity 46:957–967
Tu Z, Li Y, Dai Y et al (2017) MiR-140/BDNF axis regulates normal human astrocyte proliferation and LPS-induced IL-6 and TNF-alpha secretion. Biomed Pharmacother 91:899–905
Cui QL, Almazan G (2007) IGF-I-induced oligodendrocyte progenitor proliferation requires PI3K/Akt, MEK/ERK, and Src-like tyrosine kinases. J Neurochem 100:1480–1493
Lu Y, Lei S, Wang N et al (2016) Protective effect of minocycline against ketamine-induced injury in neural stem cell: involvement of PI3K/Akt and Gsk-3 Beta pathway. Front Mol Neurosci 9:135
Grimes CA, Jope RS (2001) The multifaceted roles of glycogen synthase kinase 3beta in cellular signaling. Prog Neurobiol 65:391–426
Jung EM, Ka M, Kim WY (2016) Loss of GSK-3 causes abnormal Astrogenesis and behavior in mice. Mol Neurobiol 53:3954–3966
Polyak K, Kato JY, Solomon MJ et al (1994) p27Kip1, a cyclin-Cdk inhibitor, links transforming growth factor-beta and contact inhibition to cell cycle arrest. Genes Dev 8:9–22
Ding S (2014) Dynamic reactive astrocytes after focal ischemia. Neural Regen Res 9:2048–2052
Robel S (2017) Astroglial scarring and seizures: a cell biological perspective on epilepsy. Neuroscientist 23:152–168
Curia G, Lucchi C, Vinet J et al (2014) Pathophysiogenesis of mesial temporal lobe epilepsy: is prevention of damage antiepileptogenic? Curr Med Chem 21:663–688
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156
Nigg EA (1995) Cyclin-dependent protein kinases: key regulators of the eukaryotic cell cycle. Bioessays 17:471–480
Malumbres M, Barbacid M (2001) To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 1:222–231
Nakayama KI, Hatakeyama S, Nakayama K (2001) Regulation of the cell cycle at the G1-S transition by proteolysis of cyclin E and p27Kip1. Biochem Biophys Res Commun 282:853–860
Di Giovanni S, Movsesyan V, Ahmed F et al (2005) Cell cycle inhibition provides neuroprotection and reduces glial proliferation and scar formation after traumatic brain injury. Proc Natl Acad Sci U S A 102:8333–8338
Zhu Z, Zhang Q, Yu Z et al (2007) Inhibiting cell cycle progression reduces reactive astrogliosis initiated by scratch injury in vitro and by cerebral ischemia in vivo. Glia 55:546–558
Choi JS, Park HJ, Kim HY et al (2005) Phosphorylation of PTEN and Akt in astrocytes of the rat hippocampus following transient forebrain ischemia. Cell Tissue Res 319:359–366
Diehl JA, Cheng M, Roussel MF et al (1998) Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis and subcellular localization. Genes Dev 12:3499–3511
Yu XJ, Han QB, Wen ZS et al (2012) Gambogenic acid induces G1 arrest via GSK3beta-dependent cyclin D1 degradation and triggers autophagy in lung cancer cells. Cancer Lett 322:185–194
Tseng AS, Engel FB, Keating MT (2006) The GSK-3 inhibitor BIO promotes proliferation in mammalian cardiomyocytes. Chem Biol 13:957–963
Acknowledgements
XL and CD designed the study. CD, SW, SZ, and XL performed the experiments. SW, SS, and WD analyzed the data. CD, SZ, PH, QC, TG, WC and WL arranged the Figs. CD and XL wrote the draft, SZ and SS revised the draft. All the authors approved the final version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (81871021, 81471209, 81641055) and the National Science and Technology Major Project (2017ZX09304018).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors claim no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dong, C., Wen, S., Zhao, S. et al. Salidroside Inhibits Reactive Astrogliosis and Glial Scar Formation in Late Cerebral Ischemia via the Akt/GSK-3β Pathway. Neurochem Res 46, 755–769 (2021). https://doi.org/10.1007/s11064-020-03207-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-020-03207-8