Abstract
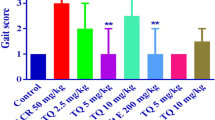
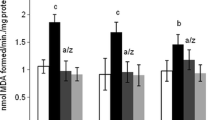
The present study evaluated biochemical endpoints characterizing acrylamide (ACR) neurotoxicity in the cortex of rats, following the possible neuroprotective activity of thymoquinone (TQ), an active constituent of Nigella sativa. ACR (50 mg/kg, intraperitoneal [i.p.]) concurrently with TQ (2.5, 5 and 10 mg/kg, i.p.) for 11 days were administered to rats. As positive control, vitamin E was used. After 11 days of injections, narrow beam test (NBT) was performed. The levels of reduced glutathione (GSH) and malondialdehyde (MDA) were measured and Western blotting was done for mitogen-activated protein kinases (MAPKinases) and apoptosis pathways proteins in the rats’ cortex. Additionally, Evans blue assay was done to evaluate the integrity of blood brain barrier (BBB). Administration of ACR significantly induced gait abnormalities. A significant decrease and increase in the levels of GSH and MDA was observed in the cortex of ACR-treated rats, respectively. The elevation in the levels of caspases 3 and 9, glial fibrillary acidic protein (GFAP) content, and Bax/Bcl-2, P-P38/P38 and P-JNK/JNK ratios accompanied by reduction in myelin basic protein (MBP) content and P-ERK/ERK ratio were noticed in the ACR group. TQ (5 mg/kg) improved gait abnormalities, and restored these changes. ACR affected the integrity of BBB while TQ was able to maintain the integrity of this barrier. TQ reversed the alterations in the protein contents of MAP kinase and apoptosis signaling pathways as well as MBP and GFAP contents, induced by ACR. It protected against ACR-mediated neurotoxicity, partly through its antioxidant and antiapoptotic properties.











Similar content being viewed by others
References
Morales G et al (2014) Effect of natural extracts on the formation of acrylamide in fried potatoes. LWT-Food Sci Technol 58(2):587–593
Smith EA, Oehme FW (1991) Acrylamide and polyacrylamide: a review of production, use, environmental fate and neurotoxicity. Rev Environ Health 9(4):215–228
Mottram DS, Wedzicha BL, Dodson AT (2002) Acrylamide is formed in the Maillard reaction. Nature 419(6906):448–449
Erkekoglu P, Baydar T (2014) Acrylamide neurotoxicity. Nutr Neurosci 17(2):49–57
Abelli L et al (1991) Acrylamide-induced visceral neuropathy: evidence for the involvement of capsaicin-sensitive nerves of the rat urinary bladder. Neuroscience 41(1):311–321
Spencer PS, Schaumburg HH (1974) A review of acrylamide neurotoxicity. Part II. Experimental animal neurotoxicity and pathologic mechanisms. Can J Neurol Sci 1(3):152–169
LoPachin RM et al (2002) Neurological evaluation of toxic axonopathies in rats: acrylamide and 2,5-hexanedione. Neurotoxicology 23(1):95–110
Cao J et al (2008) Curcumin attenuates acrylamide-induced cytotoxicity and genotoxicity in HepG2 cells by ROS scavenging. J Agric Food Chem 56(24):12059–12063
LoPachin RM, Balaban CD, Ross JF (2003) Acrylamide axonopathy revisited. Toxicol Appl Pharmacol 188(3):135–153
LoPachin RM, Barber DS, Gavin T (2008) Molecular mechanisms of the conjugated alpha, beta-unsaturated carbonyl derivatives: relevance to neurotoxicity and neurodegenerative diseases. Toxicol Sci 104(2):235–249
Lee JG, Wang YS, Chou CC (2014) Acrylamide-induced apoptosis in rat primary astrocytes and human astrocytoma cell lines. Toxicol In Vitro 28(4):562–570
Kim EK, Choi EJ (2015) Compromised MAPK signaling in human diseases: an update. Arch Toxicol 89(6):867–882
Ho PJ, Chou CK, Yeh SF (2012) Role of JNK and p38 MAPK in Taiwanin A-induced cell death. Life Sci 91(25–26):1358–1365
Newhouse K et al (2004) Rotenone-induced apoptosis is mediated by p38 and JNK MAP kinases in human dopaminergic SH-SY5Y cells. Toxicol Sci 79(1):137–146
Pan X et al (2017) Mitochondrion-mediated apoptosis induced by acrylamide is regulated by a balance between Nrf2 antioxidant and MAPK signaling pathways in PC12 Cells. Mol Neurobiol 54(6):4781–4794
Tabeshpour J et al (2019) Neuroprotective effects of thymoquinone in acrylamide-induced peripheral nervous system toxicity through MAPKinase and apoptosis pathways in rat. Neurochem Res 44(5):1101–1112
Lakshmi D et al (2012) Ameliorating effect of fish oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex. Neurochem Res 37(9):1859–1867
Sumizawa T, Igisu H (2007) Apoptosis induced by acrylamide in SH-SY5Y cells. Arch Toxicol 81(4):279–282
Liu Z et al (2015) Acrylamide induces mitochondrial dysfunction and apoptosis in BV-2 microglial cells. Free Radic Biol Med 84:42–53
Rodriguez-Ramiro I et al (2011) Procyanidin B2 and a cocoa polyphenolic extract inhibit acrylamide-induced apoptosis in human Caco-2 cells by preventing oxidative stress and activation of JNK pathway. J Nutr Biochem 22(12):1186–1194
Goudarzi M et al (2019) Neuroprotective effects of Ellagic acid against acrylamide-induced neurotoxicity in rats. Neurol Res 41(5):419–428
Uthra C et al (2017) Therapeutic potential of quercetin against acrylamide induced toxicity in rats. Biomed Pharmacother 86:705–714
Mohammadzadeh L, Hossein Hosseinzadeh SM (2018) Protective effect of grape seed extract against acrylamide-induced neurotoxicity in vitro and in vivo. JRPS 7:344–356
Hosseinzadeh H, Tabeshpur J, Mehri S (2014) Effect of saffron extract on acrylamide-induced toxicity: in vitro and in vivo assessment. Chin J Integr Med. In Press
Mehri S, Meshki MA, Hosseinzadeh H (2015) Linalool as a neuroprotective agent against acrylamide-induced neurotoxicity in Wistar rats. Drug Chem Toxicol 38(2):162–166
Motamedshariaty VS et al (2014) Effects of rutin on acrylamide-induced neurotoxicity. DARU J Pharm Sci 22(1):27
Mehri S et al (2014) Chrysin reduced acrylamide-induced neurotoxicity in both in vitro and in vivo assessments. Iran Biomed J 18(2):101–106
Ali BH, Blunden G (2003) Pharmacological and toxicological properties of Nigella sativa. Phytother Res 17(4):299–305
Sangi SMA et al (2015) Antihyperglycemic effect of thymoquinone and oleuropein, on streptozotocin-induced diabetes mellitus in experimental animals. Pharmacogn Mag 11(Suppl 2):S251–S257
Taka E et al (2015) Anti-inflammatory effects of thymoquinone in activated BV-2 microglial cells. J Neuroimmunol 286:5–12
Celik F et al (2014) Therapeutic effects of thymoquinone in a model of neuropathic pain. Curr Ther Res Clin E 76:11–16
Bakathir HA, Abbas NA (2010) Detection of the antibacterial effect of Nigella sativa ground seeds with water. Afr J Tradit Complement Altern Med 8(2):159–164
Hosseinzadeh H, Parvardeh S (2004) Anticonvulsant effects of thymoquinone, the major constituent of Nigella sativa seeds, in mice. Phytomedicine 11(1):56–64
Cobourne-Duval MK et al (2016) The antioxidant effects of thymoquinone in activated BV-2 murine microglial cells. Neurochem Res 41(12):3227–3238
Mehri S et al (2014) Neuroprotective effect of thymoquinone in acrylamide-induced neurotoxicity in Wistar rats. Iran J Basic Med Sci 17(12):1007–1011
Islam MH, Ahmad IZ, Salman MT (2015) Neuroprotective effects of Nigella sativa extracts during germination on central nervous system. Pharmacogn Mag 11(Suppl 1):S182–S189
Sedaghat R, Roghani M, Khalili M (2014) Neuroprotective effect of thymoquinone, the Nigella sativa bioactive compound, in 6-hydroxydopamine-induced hemi-parkinsonian rat model. Iran J Pharm Res 13(1):227–234
Al-Majed AA, Al-Omar FA, Nagi MN (2006) Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol 543(1–3):40–47
Alhebshi AH, Gotoh M, Suzuki I (2013) Thymoquinone protects cultured rat primary neurons against amyloid beta-induced neurotoxicity. Biochem Biophys Res Commun 433(4):362–367
Al-Majed AA, Al-Omar FA, Nagi MN (2006) Neuroprotective effects of thymoquinone against transient forebrain ischemia in the rat hippocampus. Eur J Pharmacol 543(1):40–47
Firdaus F et al (2019) Evaluation of phyto-medicinal efficacy of thymoquinone against arsenic induced mitochondrial dysfunction and cytotoxicity in SH-SY5Y cells. Phytomedicine 54:224–230
Ismail N et al (2013) Thymoquinone prevents beta-amyloid neurotoxicity in primary cultured cerebellar granule neurons. Cell Mol Neurobiol 33(8):1159–1169
Ullah I et al (2012) Neuroprotection with metformin and thymoquinone against ethanol-induced apoptotic neurodegeneration in prenatal rat cortical neurons. BMC Neurosci 13(1):11
El-Najjar N et al (2010) Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 15(2):183–195
Ismail N et al (2016) Modulation of hydrogen peroxide-induced oxidative stress in human neuronal cells by thymoquinone-rich fraction and thymoquinone via transcriptomic regulation of antioxidant and apoptotic signaling genes. Oxid Med Cell Longev. https://doi.org/10.1155/2016/2528935
Gumustas K et al (2007) The effects of vitamin E on lipid peroxidation, nitric oxide production and superoxide dismutase expression in hyperglycemic rats with cerebral ischemia-reperfusion injury. Turk Neurosurg 17(2):78–82
Prasad SN, Muralidhara (2014) Mitigation of acrylamide-induced behavioral deficits, oxidative impairments and neurotoxicity by oral supplements of geraniol (a monoterpene) in a rat model. Chem Biol Interact 223:27–37
Tsikas D (2017) Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: analytical and biological challenges. Anal Biochem 524:13–30
Mehri S et al (2015) Crocin reduced acrylamide-induced neurotoxicity in Wistar rat through inhibition of oxidative stress. Iran J Basic Med Sci 18(9):902–908
Xu S et al (2015) The neuroprotective effects of taurine against nickel by reducing oxidative stress and maintaining mitochondrial function in cortical neurons. Neurosci Lett 590:52–57
Moron MS, Depierre JW, Mannervik B (1979) Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta 582(1):67–78
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72(1):248–254
Belayev L et al (1996) Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res 739(1):88–96
Pennisi M et al (2013) Neurotoxicity of acrylamide in exposed workers. Int J Environ Res Public Health 10(9):3843–3854
Shukla PK et al (2002) Protective effect of Acorus calamus against acrylamide induced neurotoxicity. Phytother Res 16(3):256–260
Mousavi SH et al (2010) Protective effect of Nigella sativa extract and thymoquinone on serum/glucose deprivation-induced PC12 cells death. Cell Mol Neurobiol 30(4):591–598
Zhu Y-J et al (2008) Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res 33(11):2310
Prasad SN, Muralidhara (2013) Neuroprotective efficacy of eugenol and isoeugenol in acrylamide-induced neuropathy in rats: behavioral and biochemical evidence. Neurochem Res 38(2):330–345
Hosseinzadeh H et al (2007) Effect of thymoquinone and Nigella sativa seeds oil on lipid peroxidation level during global cerebral ischemia-reperfusion injury in rat hippocampus. Phytomedicine 14(9):621–627
Hosseini M et al (2015) Effects of the hydro-alcoholic extract of Nigella sativa on scopolamine-induced spatial memory impairment in rats and its possible mechanism. Chin J Integr Med 21(6):438–444
Erkut A et al (2016) Protective effects of thymoquinone and alpha-tocopherol on the sciatic nerve and femoral muscle due to lower limb ischemia-reperfusion injury. Eur Rev Med Pharmacol Sci 20(6):1192–1202
Chang L, Karin M (2001) Mammalian MAP kinase signalling cascades. Nature 410(6824):37–40
Xia Z et al (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270(5240):1326
Chen J-H, Chou C-C (2015) Acrylamide inhibits cellular differentiation of human neuroblastoma and glioblastoma cells. Food Chem Toxicol 82:27–35
Kim KH et al (2015) Acrylamide induces senescence in macrophages through a process involving ATF3, ROS, p38/JNK, and a telomerase-independent pathway. Chem Res Toxicol 28(1):71–86
Gokce EC et al (2016) Neuroprotective effects of thymoquinone against spinal cord ischemia-reperfusion injury by attenuation of inflammation, oxidative stress, and apoptosis. J Neurosurg Spine 24(6):949–959
Beker M, Dallı T, Elibol B (2018) Thymoquinone can improve neuronal survival and promote neurogenesis in rat hippocampal neurons. Mol Nutr Food Res 62(5):1700768
Hu J et al (2016) Exploration of Bcl-2 family and caspases-dependent apoptotic signaling pathway in Zearalenone-treated mouse endometrial stromal cells. Biochem Biophys Res Commun 476(4):553–559
Shimizu S, Narita M, Tsujimoto Y (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399(6735):483–487
Chen M et al (2007) Caspase-9-induced mitochondrial disruption through cleavage of anti-apoptotic BCL-2 family members. J Biol Chem 282(46):33888–33895
Mehri S et al (2012) Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol 32(2):227–235
Kianfar M et al (2018) The protective effect of fasudil against acrylamide-induced cytotoxicity in PC12 cells. Drug Chem Toxicol 13:1–7
Hosseini SM et al (2017) Protective effect of thymoquinone, the active constituent of Nigella sativa fixed oil, against ethanol toxicity in rats. Iran J Basic Med Sci 20(8):927–939
Pedraza L et al (1997) The active transport of myelin basic protein into the nucleus suggests a regulatory role in myelination. Neuron 18(4):579–589
Han CH (2012) Differential gene expression pattern in brains of acrylamide-administered mice. KJVR 52(2):99–104
Parng C et al (2007) Neurotoxicity assessment using zebrafish. J Pharmacol Toxicol Methods 55(1):103–112
Elgholam M et al (2015) The role of rosemary against acrylamide developmental toxicity on the white matter of the rat spinal cord. Menoufia Med J 28(3):765–773
Hol EM, Pekny M (2015) Glial fibrillary acidic protein (GFAP) and the astrocyte intermediate filament system in diseases of the central nervous system. Curr Opin Cell Biol 32:121–130
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119(1):7–35
Jany PL et al (2015) CSF and blood levels of GFAP in Alexander disease. eNeuro. https://doi.org/10.1523/ENEURO.0080-15.2015
Shi J et al (2012) Effect of sub-acute exposure to acrylamide on GABAergic neurons and astrocytes in weaning rat cerebellum. Toxicol Ind Health 28(1):10–20
Hadinia A et al (2010) The effect of Silybum marianum on GFAP and spatial memory in a mouse model of Alzheimer’s disease. Armaghane Danesh 14(4):65–75
Carpentier A et al (2016) Clinical trial of blood-brain barrier disruption by pulsed ultrasound. Sci Transl Med 8(343):343re2
Attoff K et al (2016) Acrylamide alters CREB and retinoic acid signaling pathways during differentiation of the human neuroblastoma SH-SY5Y cell line. Toxicol In Vitro 35:1879–3177
Edwards PM (1975) Neurotoxicity of acrylamide and its analogues and effects of these analogues and other agents on acrylamide neuropathy. Br J Ind Med 32(1):31
Choi J et al (2003) Vitamin E prevents oxidation of antiapoptotic proteins in neuronal cells. Proteomics 3(1):73–77
Lou H et al (2006) Adriamycin-induced oxidative stress, activation of MAP kinases and apoptosis in isolated cardiomyocytes. Pathophysiology 13(2):103–109
Acknowledgements
Authors are grateful to the Vice Chancellor of Research, Mashhad University of Medical Sciences, Mashhad, Iran for financial support. The data reported in this article are part of a Ph.D. thesis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tabeshpour, J., Mehri, S., Abnous, K. et al. Role of Oxidative Stress, MAPKinase and Apoptosis Pathways in the Protective Effects of Thymoquinone Against Acrylamide-Induced Central Nervous System Toxicity in Rat. Neurochem Res 45, 254–267 (2020). https://doi.org/10.1007/s11064-019-02908-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02908-z