Abstract
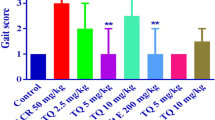
The primary objective of this investigation was to assess the neuroprotective efficacy of spice active principles namely Eugenol (Eug) and isoeugenol (IE) in an acrylamide (ACR) neuropathy model in rats. In the present study, ACR administration (50 mg/kg bw, i.p. 3 times/week) for 5 weeks to growing rats caused typical symptoms of neuropathy. We found that treatment of ACR rats with spice active principles (10 mg/kg bw, for 5 weeks) caused marked improvement in gait score and responses in a battery of behavioral tests. Terminally, both spice active principles markedly attenuated ACR-induced markers of oxidative stress viz., reactive oxygen species (ROS), malondialdehyde (MDA) and nitric oxide (NO) in sciatic nerve (SN) as well as brain regions (cortex Ct, cerebellum Cb). Treatment with Eug restored the reduced glutathione levels in SN and brain regions. Interestingly, both spice active principles effectively diminished ACR-induced elevation in cytosolic calcium levels and acetylcholinesterase activity in SN and Ct. Further, the diminished activity of ATPase among ACR rats was enhanced in SN and restored in brain regions. Furthermore, Eug treatment significantly offset ACR-induced depletion in dopamine levels in brain regions. Collectively our findings suggest the propensity of these spice active principles to attenuate ACR-induced neuropathy. Further studies are necessary to understand the precise molecular mechanism/s by which these spice active principles attenuate neuropathy. Nevertheless, our data clearly demonstrate the beneficial effects of spice active principles in ACR-induced neuropathy in rats and suggest their possible therapeutic usage as an adjuvant in the management of other forms of neuropathy in humans.









Similar content being viewed by others
Abbreviations
- CNS:
-
Central nervous system
- PNS:
-
Peripheral nervous system
- ROS:
-
Reactive oxygen species
- MDA:
-
Malondialdehyde
- HP:
-
Hydroperoxides
- NO:
-
Nitric oxide
- GSH:
-
Reduced glutathione
- SOD:
-
Superoxide dismutase
- CAT:
-
Catalase
- GST:
-
Glutathione-S-transferase
- AChE:
-
Acetylcholinesterase
References
Hidalgo ME, De la Rosa C (2009) Antioxidant capacity of eugenol derivatives. Quim Nova 32:1467–1470
Choi YC, Park KR, Lee JH et al (2007) Isoeugenol suppression of inducible nitric oxide synthase expression is mediated by down regulation of NF-kB, ERK1/2 and p38 kinase. Eur J Pharmacol 576:151–159
Mahapatra SK, Chakraborty SP, Majumdar S et al (2009) Eugenol protects nicotine induced superoxide mediated oxidative damage in murine peritoneal macrophages in vitro. Eur J Pharmacol 623:132–140
Prasad SN, Raghavendra R, Lokesh BR et al (2004) Spice phenolics inhibit human PMNL 5-lipoxygenase. Prostaglandins Leukot Essent Fat Acids 70:521–528
Wie MB, Won MH, Lee KH et al (1997) Eugenol neuronal cells from excitotoxic and oxidative injury in primary cortical cultures. Neurosci Lett 225:93–96
Kabuto H, Tada M, Kohno M (2007) Eugenol [2-methoxy-4-(2-propenyl) phenol] prevents 6-hydroxydopamine induced dopamine depression and lipid peroxidation inductivity in mouse straitum. Biol Pharm Bull 30:423–427
Baskaran Y, Vishwanathan P, Anuradha CV (2010) Investigation of antioxidant, anti-inflammatory and DNA-protective properties of eugenol in thioacetamide-induced liver injury in rats. Toxicology 268:204–212
Tardiff RG, Gargas ML, Kirman CR et al (2010) Estimation of safe dietary intake levels of acrylamide for humans. Food Chem Toxicol 48:658–667
Yener Y, Kalipci E (2009) The carcinogenic effects of acrylamide formed during cooking of some foods. Acad J Cancer Res 2:25–32
LoPachin RM, Gavin T (2008) Acrylamide induced nerve terminal damage: relevance to neurotoxic and neurodegenerative mechanisms. J Agric Food Chem 56:5994–6003
Tareke E, Rydberg P, Karlsson P et al (2002) Analysis of acrylamide, a carcinogen formed in food-stuffs. J Agric Food Chem 50:4998–5006
Sumner SC, Williams CC, Snyder RW (2003) Acrylamide: a comparison of metabolism and hemoglobin adducts in rodents following dermal, intraperitoneal, oral, or inhalation exposure. Toxicol Sci 75:260–270
Dybing E, Farmer PB, Anderson M et al (2005) Human exposure and internal dose assessments of acrylamide in food. Food Chem Toxicol 43:365–410
Zhu YJ, Zeng T, Zhu YB et al (2008) Effects of acrylamide on the nervous tissue antioxidant system and sciatic nerve electrophysiology in the rat. Neurochem Res 33:2310–2317
Ghareeb DA, Khalil ACR, Elbassoumy AM et al (2010) Ameliorated effects of garlic (Allium sativum) on biomarkers of subchronic acrylamide hepatotoxicity and brain toxicity in rats. Toxicol Environ Chem 92:1357–1372
Cook LEL Jr, Tareke E, Word B et al (2011) Food contaminant acrylamide increase expression of Cox-2 and nitric oxide synthase in breast epithelial cells. Toxicol Ind Health 27:11–18
Lim TG, Lee BK, Kwon JY et al (2011) Acrylamide up-regulates cyclooxygenase-2 expression through the MEK/ERK signalling pathway in mouse epidermal cells. Food Chem Toxicol 49:1254–1259
Lehning EJ, Balaban CD, Ross JF et al (2002) Acrylamide neuropathy: I. Spatiotemporal characteristics of nerve cell damage in rat cerebellum. NeuroToxicology 23:397–414
LoPachin RM, Balaban CD, Ross JF (2003) Acrylamide axonopathy revisited. Toxicol Appl Pharmacol 188:135–153
Ling B, Authier N, Balayssac D et al (2005) Assessment of nociception in acrylamide-induced neuropathy in rats. Pain 119:104–112
Yu S, Son F, Yu J et al (2006) Acrylamide alters cytoskeletal protein in sciatic nerve. Neurochem Res 31:1197–1204
Xie Q, Liu Y, Sun H et al (2008) Inhibition of acrylamide toxicity in mice by three dietary constituents. J Agric Food Chem 56:6054–6060
Aggarwal BB, Shishodia S (2004) Suppression of the nuclear factor- kB activation pathway by spice derived phytochemicals. Ann NY Acad Sci 1030:434–441
Hosamani R, Muralidhara (2009) Neuroprotective efficacy of Bacopa monnieri against rotenone induced oxidative stress and neurotoxicity in Drosophila melanogaster. NeuroToxicology 30:977–985
Girish C, Muralidhara (2012) Propensity of Selaginella delicatula aqueous extract to offset rotenone-induced oxidative dysfunctions and neurotoxicity in Drosophila melanogaster: implications for Parkinson’s disease. NeuroToxicology 33:444–456
Prasad SN, Muralidhara (2012) Evidence of acrylamide induced oxidative stress and neurotoxicity in Drosophila melanogaster: its amelioration with spice active enrichment: relevance to neuropathy. NeuroToxicology 33:1254–1264
Shinomol GK, Bharath MMS, Muralidhara (2012) Neuromodulatory propensity of Bacopa monnieri leaf extract against 3-nitropopionic acid induced oxidative stress: in vitro and in vivo evidences. Neurotox Res 22:102–114
Ohkawa H, Ohnishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 9:351–358
Pradat PF, Kennel P, Sadaqui SN et al (2001) Continuous delivery of neurotrophin 3by gene therapy has a neuroprotective effect in experimental models of diabetic and acrylamide neuropathies. Hum Gene Ther 12:2237–2249
Kanan SA, SACRdé NE, Haddad JJ et al (1996) Endotoxin induced local inflammation and hyperalgesia in rats and mice: a new model of inflammatory pain. Pain 66:373–379
Edwards PM, Parker VH (1977) A simple, sensitive and objective method for early assessment of acrylamide neuropathy in rats. Toxicol Appl Pharmacol 40:589–591
Sandhir R, Mehrotra A, Kamboj SS (2010) Lycopene prevents 3-nitropropionic acid induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem Int 57:579–587
Moreadith RW, Fiskum J (1984) Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem 137:360–367
Muthuraman A, Diwan V, Jaggi AS et al (2008) Ameliorative effects of Ocimim sanctum in sciatic nerve transection induced neuropathy in rats. Eur J Pharmacol 587:104–111
Shinomol GK, Muralidhara (2008) Prophylactic neuroprotective property of Centella asiatica against 3-nitropropionic acid induced oxidative stress and mitochondrial dysfunctions in brain regions of prepubertal mice. NeuroToxicology 29:948–957
Wolf SP (1994) Ferrous ion oxidation in presence of ferric ion indicator xylenol orange for measurement of hydroperoxides. Methods Enzymol 233:182–189
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Kostyuk VA, Potapovich AI (1989) Superoxide driven oxidation of quercetin and a simple sensitive assay for determination of superoxide dismutase. Biochem Int 19:1117–1124
Guthenberg C, Alin P, Mannervik B (1985) Glutathione transferase from rat testis. Methods Enzymol 113:507–510
Mokrasch LC, Teschke EJ (1984) Glutathione content of cultured cells and rodent brain regions: a specific fluorimetric assay. Anal Biochem 140:506–509
Ellmann GE, Courtney KD, Andersen V et al (1961) A new and rapid colorimetric determination. Biochem Pharmacol 13:205–208
Desaiah D, Phillips TD, Hayes AW et al (1979) Effect of aflatoxins on ATPase activities on mouse and rat liver. J Environ Sci Health B 14:265–278
Fiske CH, Subbarow YJ (1925) The colorimetric determination of phosphorous. J Biol Chem 66:375–381
Hitoshi A, Satoh T, Sakai N et al (1997) Generation of free radicals during lipid peroxide triggered apoptosis in PC 12 cells. Biochim Biophys Acta 1345:35–42
Dalpiaz A, Filosa K, de Caprariis P et al (2007) Molecular mechanism involved in the transport of a prodrug dopamine glycosyl conjugate. Int J Pharm 336:133–139
Lowry OH, Rosenbrough NJ, Farr AL et al (1951) Protein measurements using folin-phenol reagent. J Biol Chem 193:265–275
Kumar A, Kaundal RK, Iyer S et al (2007) Effects of resveratrol on nerve functions, oxidative stress and DNA fragmentation in experimental diabetic neuropathy. Life Sci 80:1236–1244
Kannappan R, Gupta SC, Kim JH et al (2011) Neuroprotection by spice derived nutraceuticals: you are what you eat? Mol Neurobiol 44:142–159
Gulcin I, Sat IG, Beydemir S et al (2004) Comparison of antioxidant activity Clove (Eugenia caryophylata Thunb) buds lavender. Food Chem 87:393–400
Lakshmi D, Gopinath K, Jayanthy G et al (2012) Ameliorating effect of fish oil on acrylamide induced oxidative stress and neuronal apoptosis in cerebral cortex. Neurochem Res 37:1859–1867
Khatun M, Eguchi S, Yamaguchi T et al (2006) Effect of Thermal Treatment on Radical-scavenging Activity of Some Spices. Food Sci Technol Res 12:178–185
Naziroglu M (2011) TRPM2 Cation channels, oxidative stress and neurological diseases: where are we now? Neurochem Res 36:355–366
Miller BA (2006) The role of TRP channels in oxidative stress-induced cell death. J Membr Biol 209:31–41
Kang KS, Yamabe N, Kim HY et al (2008) Therapeutic potential of 20(S)-ginsenoside Rg3 against streptozotocin-induced diabetic renal damage in rats. Eur J Pharmacol 591:266–272
Naziroglu M, Dikici DM, Dursun S (2012) Role of Oxidative Stress and Ca2+ Signaling on Molecular Pathways of Neuropathic Pain in Diabetes: focus on TRP Channels. Neurochem Res 37:2065–2075
Schmatz R, Mazzanti CM, Spanevello R et al (2009) Resveratrol prevents memory deficits and the increase in acetylcholinesterase activity in streptozotocin-induced diabetic rats. Eur J Pharmacol 610:42–48
Chacon MA, Reyes AE, Inestrosa NC (2003) Acetylcholinesterase induces neuronal cell loss, astrocyte hypertrophy and behavioural deficits in mammalian hippocampus. J Neurochem 87:195–204
Bernhardi RV, Alarcon R, Mezzano D et al (2005) Blood cells cholinesterase activity in early Alzeimer’s disease and vascular dementia. Dement Geriatr Cogn Disord 19:204–212
Jin QH, He HY, Shi YF et al (2004) Overexpression of acetylchoinesterase inhibited cell proliferation and promoted apoptosis in NRK cells. Acta Pharmacol Sin 25:1013–1021
Sabri MI, Spencer PS (2002) How does acrylamide perturb axon transport and induce nerve fiber degeration? Commentary on forum position paper. NeuroToxicology 23:259–263
Martyniuk CJ, Fang B, Koomen JM et al (2011) Molecular mechanism of Glyceraldehyde 3 phosphate dehydrogenase inactivation by α β unsaturated carbonyl deriavtives. Chem Res Toxicol 24:2302–2311
Zhang L, Gavin T, Barber DS et al (2011) Role of Nrf2–ARE pathway in acrylamide neurotoxicity. Toxicol Lett 205:1–7
Glass JD, Culver DG, Levey AI et al (2002) Very early activation of m-calpain in peripheral nerve during Wallerian degeneration. J Neurol Sci 196:9–20
LoPachin RM (2004) The changing view of acrylamide neurotoxicity. NeuroToxicology 25:617–630
LoPachin RM, Schwarcz AI, Gaughan CL et al (2004) In vivo and in vitro effects of acrylamide on synaptosomal neurotransmitter uptake in rat striatal synaptic vesicles. NeuroToxicology 25:349–363
LoPachin RM, Barber DS, He D et al (2006) Acrylamide inhibits dopamine uptake in rat striatal synaptic vesicles. Toxicol Sci 89:224–234
Tareke E, Lyn-Cook BD, Duhart H et al (2009) Acrylamide decreased dopamine levels and increased 3-nitrotyrosine (3-NT) levels in PC 12 cells. Neurosci Lett 458:89–92
Acknowledgments
We wish to thank the Director, CFTRI, for his keen interest in this area of research. Also, the grant from the Department of Science and Technology (Women Scientist Scheme WOS-A) New Delhi, Government of India (SR/WOS-A/LS-201/2008) to the first author is greatly acknowledged.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasad, S.N., Muralidhara Neuroprotective Efficacy of Eugenol and Isoeugenol in Acrylamide-Induced Neuropathy in rats: Behavioral and Biochemical evidence. Neurochem Res 38, 330–345 (2013). https://doi.org/10.1007/s11064-012-0924-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-012-0924-9