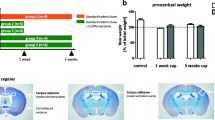
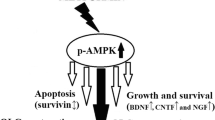
Abstract
Cuprizone (cup) model targets oligodendrocytes (OLGs) degeneration and is frequently used for the mechanistic understanding of de- and remyelination. Improperly, this classic model is time-consuming and the extent of brain lesions and behavioral deficits are changeable (both temporally and spatially) within a mouse strain. We aimed to offer an alternative, less time-consuming, and more reproducible cup model. Mice (C57BL/6) were treated with cup (400 mg kg−1 day−1/gavage) for three consecutive weeks to induce OLGs degeneration with or without YM155 (1 mg kg−1 day−1) to examine the effects of this molecule in cup neurotoxicity. Co-administration of cup and YM155 (cuYM) accelerated the intrinsic apoptosis of mature OLGs (MOG positive cells) through the upregulation of caspase-9 and caspase-3. In addition to the stimulation of oxidative stress via reduction of glutathione peroxidase and induction of malondialdehyde, behavioral deficits in both Open-field and Rota-rod tests were worsened by cuYM. In the cuYM group, the expression of BIRC5, BIRC4 and NAIP was reduced, but no significant changes were observed in the abundance of the other inhibitor of apoptosis proteins (cIAP1 and cIAP2) in comparison with the cup group. Moreover, in silico analysis validated that YM155 directly interrupts the binding sites of certain transcription factors, such as krüppel-like family (Klf), specificity proteins (SPs), myeloid zinc fingers (MZFs), zinc finger proteins (ZNFPs), and transcription factor activating enhancer-binding proteins (TFAPs), on the promoters of target genes. In conclusion, this modified model promotes cup-induced redox and apoptosis signaling, elevates behavioral deficits, saves time, minimizes variations, and can be employed for early evaluation of novel neuroprotective agents in oligodendropathies.








Similar content being viewed by others
References
Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H (2000) Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol 47(6):707–717
Sanadgol N, Zahedani SS, Sharifzadeh M, Khalseh R, Barbari GR, Abdollahi M (2017) Recent updates in imperative natural compounds for healthy brain and nerve function: a systematic review of implications for multiple sclerosis. Curr Drug Targets 18(13):1499–1517. https://doi.org/10.2174/1389450118666161108124414
Sanadgol N, Golab F, Mostafaie A, Mehdizadeh M, Abdollahi M, Sharifzadeh M, Ravan H (2016) Ellagic acid ameliorates cuprizone-induced acute CNS inflammation via restriction of microgliosis and down-regulation of CCL2 and CCL3 pro-inflammatory chemokines. Cell Mol Biol 62(12):24–30. https://doi.org/10.14715/cmb/2016.62.12.5
Abakumova TO, Kuz'kina AA, Zharova ME, Pozdeeva DA, Gubskii IL, Shepeleva II, Antonova OM, Nukolova NV, Kekelidze ZI, Chekhonin VP (2015) Cuprizone model as a tool for preclinical studies of the efficacy of multiple sclerosis diagnosis and therapy. Bull Exp Biol Med 159(1):111–115. https://doi.org/10.1007/s10517-015-2903-z
Heckers S, Held N, Kronenberg J, Skripuletz T, Bleich A, Gudi V, Stangel M (2017) Investigation of cuprizone inactivation by temperature. Neurotox Res 31(4):570–577. https://doi.org/10.1007/s12640-017-9704-2
Benardais K, Kotsiari A, Skuljec J, Koutsoudaki PN, Gudi V, Singh V, Vulinovic F, Skripuletz T, Stangel M (2013) Cuprizone [bis(cyclohexylidenehydrazide)] is selectively toxic for mature oligodendrocytes. Neurotox Res 24(2):244–250. https://doi.org/10.1007/s12640-013-9380-9
Oberoi-Khanuja TK, Murali A, Rajalingam K (2013) IAPs on the move: role of inhibitors of apoptosis proteins in cell migration. Cell Death Dis 4:e784. https://doi.org/10.1038/cddis.2013.311
Abdel-Magid AF (2017) Modulation of the inhibitors of apoptosis proteins (IAPs) activities for cancer treatment. ACS Med Chem Lett 8(5):471–473. https://doi.org/10.1021/acsmedchemlett.7b00148
Silke J, Meier P (2013) Inhibitor of apoptosis (IAP) proteins-modulators of cell death and inflammation. Cold Spring Harb Perspect Biol 1:1. https://doi.org/10.1101/cshperspect.a008730
Varughese RK, Torp SH (2016) Survivin and gliomas: a literature review. Oncol Lett 12(3):1679–1686. https://doi.org/10.3892/ol.2016.4867
Xia Z, Friedlander RM (2017) Minocycline in multiple sclerosis—compelling results but too early to tell. N Engl J Med 376(22):2191–2193. https://doi.org/10.1056/NEJMe1703230
Hebb AL, Moore CS, Bhan V, Campbell T, Fisk JD, Robertson HA, Thorne M, Lacasse E, Holcik M, Gillard J, Crocker SJ, Robertson GS (2008) Expression of the inhibitor of apoptosis protein family in multiple sclerosis reveals a potential immunomodulatory role during autoimmune mediated demyelination. Mult Scler 14(5):577–594. https://doi.org/10.1177/1352458507087468
Cheung CH, Cheng L, Chang KY, Chen HH, Chang JY (2011) Investigations of survivin: the past, present and future. Front Biosci (Landmark Ed) 16:952–961
Zhen W, Liu A, Lu J, Zhang W, Tattersall D, Wang J (2017) An alternative cuprizone-induced demyelination and remyelination mouse model. ASN Neuro 1:1. https://doi.org/10.1177/1759091417725174
Guo K, Huang P, Xu N, Xu P, Kaku H, Zheng S, Xu A, Matsuura E, Liu C, Kumon H (2015) A combination of YM-155, a small molecule survivin inhibitor, and IL-2 potently suppresses renal cell carcinoma in murine model. Oncotarget 6(25):21137–21147. https://doi.org/10.18632/oncotarget.4121
Sanadgol N, Golab F, Mostafaie A, Mehdizadeh M, Khalseh R, Mahmoudi M, Abdollahi M, Vakilzadeh G, Taghizadeh G, Sharifzadeh M (2018) Low, but not high, dose triptolide controls neuroinflammation and improves behavioral deficits in toxic model of multiple sclerosis by dampening of NF-kappaB activation and acceleration of intrinsic myelin repair. Toxicol Appl Pharmacol 342:86–98. https://doi.org/10.1016/j.taap.2018.01.023
Poorebrahim M, Asghari M, Abazari MF, Askari H, Sadeghi S, Taheri-Kafrani A, Nasr-Esfahani MH, Ghoraeian P, Aleagha MN, Arab SS, Kennedy D, Montaseri A, Mehranfar M, Sanadgol N (2018) Immunomodulatory effects of a rationally designed peptide mimetic of human IFNbeta in EAE model of multiple sclerosis. Prog Neuropsychopharmacol Biol Psychiatry 82:49–61. https://doi.org/10.1016/j.pnpbp.2017.11.028
Sanadgol N, Golab F, Askari H, Moradi F, Ajdary M, Mehdizadeh M (2018) Alpha-lipoic acid mitigates toxic-induced demyelination in the corpus callosum by lessening of oxidative stress and stimulation of polydendrocytes proliferation. Metab Brain Dis 33(1):27–37. https://doi.org/10.1007/s11011-017-0099-9
Keshavarz-Bahaghighat H, Sepand MR, Ghahremani MH, Aghsami M, Sanadgol N, Omidi A, Bodaghi-Namileh V, Sabzevari O (2018) Acetyl-L-Carnitine Attenuates Arsenic-Induced Oxidative Stress and Hippocampal Mitochondrial Dysfunction. Biol Trace Elem Res 184(2):422–435. https://doi.org/10.1007/s12011-017-1210-0
Nakahara T, Kita A, Yamanaka K, Mori M, Amino N, Takeuchi M, Tominaga F, Kinoyama I, Matsuhisa A, Kudou M, Sasamata M (2011) Broad spectrum and potent antitumor activities of YM155, a novel small-molecule survivin suppressant, in a wide variety of human cancer cell lines and xenograft models. Cancer Sci 102(3):614–621. https://doi.org/10.1111/j.1349-7006.2010.01834.x
Shirazi MK, Azarnezhad A, Abazari MF, Poorebrahim M, Ghoraeian P, Sanadgol N, Bokharaie H, Heydari S, Abbasi A, Kabiri S, Aleagha MN, Enderami SE, Dashtaki AS, Askari H (2019) The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: Involvement of oxidative stress, autophagy and apoptosis. J Cell Physiol 234(7):11411–11423. https://doi.org/10.1002/jcp.27797
Ranjbar A, Ghahremani MH, Sharifzadeh M, Golestani A, Ghazi-Khansari M, Baeeri M, Abdollahi M (2010) Protection by pentoxifylline of malathion-induced toxic stress and mitochondrial damage in rat brain. Hum Exp Toxicol 29(10):851–864. https://doi.org/10.1177/0960327110363836
Pourkhalili N, Hosseini A, Nili-Ahmadabadi A, Hassani S, Pakzad M, Baeeri M, Mohammadirad A, Abdollahi M (2011) Biochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. World J Diabetes 2(11):204–210. https://doi.org/10.4239/wjd.v2.i11.204
von Leden RE, Yauger YJ, Khayrullina G, Byrnes KR (2017) Central nervous system injury and nicotinamide adenine dinucleotide phosphate oxidase: oxidative stress and therapeutic targets. J Neurotrauma 34(4):755–764. https://doi.org/10.1089/neu.2016.4486
Bodaghi-Namileh V, Sepand MR, Omidi A, Aghsami M, Seyednejad SA, Kasirzadeh S, Sabzevari O (2018) Acetyl-l-carnitine attenuates arsenic-induced liver injury by abrogation of mitochondrial dysfunction, inflammation, and apoptosis in rats. Environ Toxicol Pharmacol 58:11–20. https://doi.org/10.1016/j.etap.2017.12.005
Mohammadi H, Karimi G, Seyed Mahdi R, Ahmad Reza D, Shafiee H, Nikfar S, Baeeri M, Sabzevari O, Abdollahi M (2011) Benefit of nanocarrier of magnetic magnesium in rat malathion-induced toxicity and cardiac failure using non-invasive monitoring of electrocardiogram and blood pressure. Toxicol Ind Health 27(5):417–429. https://doi.org/10.1177/0748233710387634
Gawryluk JW, Wang JF, Andreazza AC, Shao L, Young LT (2011) Decreased levels of glutathione, the major brain antioxidant, in post-mortem prefrontal cortex from patients with psychiatric disorders. Int J Neuropsychopharmacol 14(1):123–130. https://doi.org/10.1017/S1461145710000805
Sanadgol N, Golab F, Tashakkor Z, Taki N, Moradi Kouchi S, Mostafaie A, Mehdizadeh M, Abdollahi M, Taghizadeh G, Sharifzadeh M (2017) Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharm Biol 55(1):1679–1687. https://doi.org/10.1080/13880209.2017.1319867
Qian ZM, Li H, Sun H, Ho K (2002) Targeted drug delivery via the transferrin receptor-mediated endocytosis pathway. Pharmacol Rev 54(4):561–587
Lotocki G, Keane RW (2002) Inhibitors of apoptosis proteins in injury and disease. IUBMB Life 54(5):231–240. https://doi.org/10.1080/15216540215675
Kita A, Mitsuoka K, Kaneko N, Nakata M, Yamanaka K, Jitsuoka M, Miyoshi S, Noda A, Mori M, Nakahara T, Sasamata M (2012) Sepantronium bromide (YM155) enhances response of human B-cell non-Hodgkin lymphoma to rituximab. J Pharmacol Exp Ther 343(1):178–183. https://doi.org/10.1124/jpet.112.195925
Glaros TG, Stockwin LH, Mullendore ME, Smith B, Morrison BL, Newton DL (2012) The "survivin suppressants" NSC 80467 and YM155 induce a DNA damage response. Cancer Chemother Pharmacol 70(1):207–212. https://doi.org/10.1007/s00280-012-1868-0
Kaneko N, Mitsuoka K, Amino N, Yamanaka K, Kita A, Mori M, Miyoshi S, Kuromitsu S (2014) Combination of YM155, a survivin suppressant, with bendamustine and rituximab: a new combination therapy to treat relapsed/refractory diffuse large B-cell lymphoma. Clin Cancer Res 20(7):1814–1822. https://doi.org/10.1158/1078-0432.CCR-13-2707
Giaccone G, Zatloukal P, Roubec J, Floor K, Musil J, Kuta M, van Klaveren RJ, Chaudhary S, Gunther A, Shamsili S (2009) Multicenter phase II trial of YM155, a small-molecule suppressor of survivin, in patients with advanced, refractory, non-small-cell lung cancer. J Clin Oncol 27(27):4481–4486. https://doi.org/10.1200/JCO.2008.21.1862
Lewis KD, Samlowski W, Ward J, Catlett J, Cranmer L, Kirkwood J, Lawson D, Whitman E, Gonzalez R (2011) A multi-center phase II evaluation of the small molecule survivin suppressor YM155 in patients with unresectable stage III or IV melanoma. Invest New Drugs 29(1):161–166. https://doi.org/10.1007/s10637-009-9333-6
Papadopoulos KP, Lopez-Jimenez J, Smith SE, Steinberg J, Keating A, Sasse C, Jie F, Thyss A (2016) A multicenter phase II study of sepantronium bromide (YM155) plus rituximab in patients with relapsed aggressive B-cell Non-Hodgkin lymphoma. Leuk Lymphoma 57(8):1848–1855. https://doi.org/10.3109/10428194.2015.1113275
Satoh T, Okamoto I, Miyazaki M, Morinaga R, Tsuya A, Hasegawa Y, Terashima M, Ueda S, Fukuoka M, Ariyoshi Y, Saito T, Masuda N, Watanabe H, Taguchi T, Kakihara T, Aoyama Y, Hashimoto Y, Nakagawa K (2009) Phase I study of YM155, a novel survivin suppressant, in patients with advanced solid tumors. Clin Cancer Res 15(11):3872–3880. https://doi.org/10.1158/1078-0432.CCR-08-1946
Tolcher AW, Mita A, Lewis LD, Garrett CR, Till E, Daud AI, Patnaik A, Papadopoulos K, Takimoto C, Bartels P, Keating A, Antonia S (2008) Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J Clin Oncol 26(32):5198–5203. https://doi.org/10.1200/JCO.2008.17.2064
Steelman AJ, Zhou Y, Koito H, Kim S, Payne HR, Lu QR, Li J (2016) Activation of oligodendroglial Stat3 is required for efficient remyelination. Neurobiol Dis 91:336–346. https://doi.org/10.1016/j.nbd.2016.03.023
Cheng Q, Ling X, Haller A, Nakahara T, Yamanaka K, Kita A, Koutoku H, Takeuchi M, Brattain MG, Li F (2012) Suppression of survivin promoter activity by YM155 involves disruption of Sp1-DNA interaction in the survivin core promoter. Int J Biochem Mol Biol 3(2):179–197
Nakamura N, Yamauchi T, Hiramoto M, Yuri M, Naito M, Takeuchi M, Yamanaka K, Kita A, Nakahara T, Kinoyama I, Matsuhisa A, Kaneko N, Koutoku H, Sasamata M, Yokota H, Kawabata S (2012) Furuichi K (2012) Interleukin enhancer-binding factor 3/NF110 is a target of YM155, a suppressant of survivin. Mol Cell Proteomics 1:1. https://doi.org/10.1074/mcp.M111.013243
Ho SH, Ali A, Chin TM, Go ML (2016) Dioxonaphthoimidazoliums AB1 and YM155 disrupt phosphorylation of p50 in the NF-κB pathway. Oncotarget 7(10):11625–11636
Yamauchi T, Nakamura N, Hiramoto M, Yuri M, Yokota H, Naitou M, Takeuchi M, Yamanaka K, Kita A, Nakahara T, Kinoyama I, Matsuhisa A, Kaneko N, Koutoku H, Sasamata M, Kobori M, Katou M, Tawara S, Kawabata S, Furuichi K (2012) Sepantronium bromide (YM155) induces disruption of the ILF3/p54(nrb) complex, which is required for survivin expression. Biochem Biophys Res Commun 425(4):711–716. https://doi.org/10.1016/j.bbrc.2012.07.103
Goldberg J, Clarner T, Beyer C, Kipp M (2015) Anatomical Distribution of Cuprizone-Induced Lesions in C57BL6 Mice. J Mol Neurosci 57(2):166–175. https://doi.org/10.1007/s12031-015-0595-5
Acknowledgements
The authors are grateful to all respected research staffs in the Pharmaceutical Science Research Center, Tehran University of Medical Sciences, Tehran, Iran, for their help with the study.
Funding
This study was funded by University of Zabol (UOZ-GR-9517–13).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have no conflicts of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reiszadeh-Jahromi, S., Sepand, MR., Ramezani-sefidar, S. et al. Sepantronium Bromide (YM155), A Small Molecule Survivin Inhibitor, Promotes Apoptosis by Induction of Oxidative Stress, Worsens the Behavioral Deficits and Develops an Early Model of Toxic Demyelination: In Vivo and In-Silico Study. Neurochem Res 44, 2482–2498 (2019). https://doi.org/10.1007/s11064-019-02865-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02865-7