Abstract
Purpose
Oligodendrocytes (OLGs) damage and myelin distraction is considered as a critical step in many neurological disorders especially multiple sclerosis (MS). Cuprizone (cup) animal model of MS targets OLGs degeneration and frequently used to the mechanistic understanding of de- and remyelination. The aim of this study was exploring the effects of metformin on the OLGs regeneration, myelin repair and profile of neurotrophic factors in the mice brain after cup-induced acute demyelination.
Methods
Mice (C57BL/6 J) were fed with chow containing 0.2% cup for 5 weeks to induce specific OLGs degeneration and acute demyelination. Next, the cup was withdrawn to allow one-week recovery (spontaneous remyelination). At the end of this period, mature OLGs markers, myelin-associated neurite outgrowth inhibitor protein A (NogoA), premature specific OLGs transcription factor (Olig2), anti-apoptosis marker (survivin), neurotrophic factors, and AMPK activation were monitored in the presence or absence of metformin (50 mg/kg body weight/day) in the corpus callosum (CC).
Results
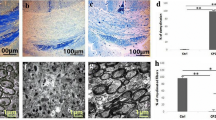
Our finding indicated that consumption of metformin during the recovery period potentially induced an active form of AMPK (p-AMPK) and promoted repopulation of mature OLGs (MOG+ cells, MBP+ cells) in CC through up-regulation of BDNF, CNTF, and NGF as well as down-regulation of NogoA and recruitment of Olig2+ precursor cells.
Conclusions
This study for the first time reveals that metformin-induced AMPK, a master regulator of energy homeostasis, activation following toxic demyelination could potentially accelerate regeneration and supports spontaneous demyelination. These findings suggest the development of new therapeutic strategies based on AMPK activation for MS in the near future.

An overview of the possible molecular mechanisms of action of metformin-mediated remyelinationa






Similar content being viewed by others
Data availability
Materials described in the manuscript, including all relevant raw data, will be freely available to any scientist wishing to use them for non-commercial purposes, without breaching.
References
Sanadgol N, Zahedani SS, Sharifzadeh M, Khalseh R, Barbari GR, Abdollahi M. Recent updates in imperative natural compounds for healthy brain and nerve function: a systematic review of implications for multiple sclerosis. Curr Drug Targets. 2017;18:1499–517.
Praet J, Guglielmetti C, Berneman Z, Van der Linden A, Ponsaerts P. Cellular and molecular neuropathology of the cuprizone mouse model: clinical relevance for multiple sclerosis. Neurosci Biobehav Rev. 2014;47:485–505.
Kochanowski J, Uchman D, Litwiniuk A, et al. Assessment of plasma brain-derived neurotrophic factor (BDNF), activity-dependent neurotrophin protein (ADNP) and vasoactive intestinal peptide (VIP) concentrations in treatment-naive humans with multiple sclerosis. Neuro Endocrinol Lett. 2015;36:148–52.
Chaldakov GN, Tonchev AB, Aloe L. NGF and BDNF: from nerves to adipose tissue, from neurokines to metabokines. Riv Psichiatr. 2009;44:79–87.
Hristova MG. Metabolic syndrome and neurotrophins: effects of metformin and non-steroidal anti-inflammatory drug treatment. Eur J Med. 2011;43:141–5.
Acosta CM, Cortes C, MacPhee H, Namaka MP. Exploring the role of nerve growth factor in multiple sclerosis: implications in myelin repair. CNS Neurol Disord Drug Targets. 2013;12:1242–56.
Xiao J, Wong AW, Willingham MM, van den Buuse M, Kilpatrick TJ, Murray SS. Brain-derived neurotrophic factor promotes central nervous system myelination via a direct effect upon oligodendrocytes. Neurosignals. 2010;18:186–202.
Khorshid AT, Acosta C, Cortes C, Lakowski TM, Gangadaran S, Namaka M. Transcriptional regulation of BDNF by methyl CpG binding protein 2 (MeCP2): a novel mechanism for re-myelination and/or myelin repair involved in the treatment of multiple sclerosis (MS). Mol Neurobiol. 2016;53:1092–107.
Pasquin S, Sharma M, Gauchat JF. Ciliary neurotrophic factor (CNTF): new facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev. 2015;26:507–15.
Linker RA, Maurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–4.
Stankoff B, Aigrot MS, Noel F, Wattilliaux A, Zalc B, Lubetzki C. Ciliary neurotrophic factor (CNTF) enhances myelin formation: a novel role for CNTF and CNTF-related molecules. J Neurosci. 2002;22:9221–7.
Kuhlmann T, Remington L, Cognet I, Bourbonniere L, Zehntner S, Guilhot F, et al. Continued administration of ciliary neurotrophic factor protects mice from inflammatory pathology in experimental autoimmune encephalomyelitis. Am J Pathol. 2006;169:584–98.
Sui YP, Zhang XX, Lu JL, Sui F. New insights into the roles of Nogo-A in CNS biology and diseases. Neurochem Res. 2015;40:1767–85.
Varughese RK, Torp SH. Survivin and gliomas: a literature review. Oncol Lett. 2016;12:1679–86.
Wang J, Gallagher D, DeVito LM, et al. Metformin activates an atypical PKC-CBP pathway to promote neurogenesis and enhance spatial memory formation. Cell Stem Cell. 2012;11:23–35.
Smieszek A, Strek Z, Kornicka K, Grzesiak J, Weiss C, Marycz K. Antioxidant and anti-senescence effect of metformin on mouse olfactory ensheathing cells (mOECs) may be associated with increased brain-derived neurotrophic factor levels-an ex vivo study. Int J Mol Sci. 2017;18:E872.
Nath N, Khan M, Paintlia MK, Hoda MN, Giri S. Metformin attenuated the autoimmune disease of the central nervous system in animal models of multiple sclerosis. J Immunol. 2009;182:8005–14.
Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, et al. Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol. 2014;171:3146–57.
Buzzai M, Jones RG, Amaravadi RK, Lum JJ, DeBerardinis RJ, Zhao F, et al. Systemic treatment with the antidiabetic drug metformin selectively impairs p53-deficient tumor cell growth. Cancer Res. 2007;67:6745–52.
Sanadgol N, Golab F, Mostafaie A, Mehdizadeh M, Abdollahi M, Sharifzadeh M, et al. Ellagic acid ameliorates cuprizone-induced acute CNS inflammation via restriction of microgliosis and down-regulation of CCL2 and CCL3 pro-inflammatory chemokines. Cell Mol Biol (Noisy-le-grand). 2016;62:24–30.
Sanchooli J, Ramroodi N, Sanadgol N, Sarabandi V, Ravan H, Rad RS. Relationship between metalloproteinase 2 and 9 concentrations and soluble CD154 expression in Iranian patients with multiple sclerosis. Kaohsiung J Med Sci. 2014;30:235–42.
Poorebrahim M, Asghari M, Abazari MF, Askari H, Sadeghi S, Taheri-Kafrani A, et al. Immunomodulatory effects of a rationally designed peptide mimetic of human IFNβ in EAE model of multiple sclerosis. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;82:49–61.
Sanadgol N, Golab F, Mostafaie A, Mehdizadeh M, Khalseh R, Mahmoudi M, et al. Low, but not high, dose triptolide controls neuroinflammation and improves behavioral deficits in toxic model of multiple sclerosis by dampening of NF-κB activation and acceleration of intrinsic myelin repair. Toxicol Appl Pharmacol. 2018;342:86–98.
Keshavarz-Bahaghighat H, Sepand MR, Ghahremani MH, Aghsami M, Sanadgol N, Omidi A, et al. Acetyl-l-carnitine attenuates arsenic-induced oxidative stress and hippocampal mitochondrial dysfunction. Biol Trace Elem Res. 2018;184:422–35.
Gibson EM, Geraghty AC, Monje M. Bad wrap: myelin and myelin plasticity in health and disease. Dev Neurobiol. 2017;78:123–35.
Jiang H, Tian KW, Zhang F, Wang B, Han S. Reg-2, a downstream signaling protein in the ciliary neurotrophic factor survival pathway, alleviates experimental autoimmune encephalomyelitis. Front Neuroanat. 2016;10:50.
Yang T, Zheng Q, Wang S, Fang L, Liu L, Zhao H, et al. Effect of catalpol on remyelination through experimental autoimmune encephalomyelitis acting to promote Olig1 and Olig2 expressions in mice. BMC Complement Altern Med. 2017;17:240.
Sanadgol N, Golab F, Tashakkor Z, Taki N, Moradi Kouchi S, Mostafaie A, et al. Neuroprotective effects of ellagic acid on cuprizone-induced acute demyelination through limitation of microgliosis, adjustment of CXCL12/IL-17/IL-11 axis and restriction of mature oligodendrocytes apoptosis. Pharm Biol. 2017;55:1679–87.
Sanadgol N, Golab F, Askari H, Moradi F, Ajdary M, Mehdizadeh M. Alpha-lipoic acid mitigates toxic-induced demyelination in the corpus callosum by lessening of oxidative stress and stimulation of polydendrocytes proliferation. Metab Brain Dis. 2018;33:27–37.
Khodanovich MY, Pishchelko AO, Glazacheva VY, Pan ES, Krutenkova EP, Trusov VB, et al. Plant polyprenols reduce demyelination and recover impaired oligodendrogenesis and neurogenesis in the cuprizone murine model of multiple sclerosis. Phytother Res. 2019;33:1363–73.
Paintlia AS, Paintlia MK, Mohan S, Singh AK, Singh I. AMP-activated protein kinase signaling protects oligodendrocytes that restore central nervous system functions in an experimental autoimmune encephalomyelitis model. Am J Pathol. 2013;183:526–41.
Razavi S, Nazem G, Mardani M, Esfandiari E, Salehi H, Esfahani SH. Neurotrophic factors and their effects in the treatment of multiple sclerosis. Adv Biomed Res. 2015;4:53.
Cao Q, He Q, Wang Y, Cheng X, Howard RM, Zhang Y, et al. Transplantation of ciliary neurotrophic factor-expressing adult oligodendrocyte precursor cells promotes remyelination and functional recovery after spinal cord injury. J Neurosci. 2010;30:2989–3001.
Vernerey J, Macchi M, Magalon K, Cayre M, Durbec P. Ciliary neurotrophic factor controls progenitor migration during remyelination in the adult rodent brain. J Neurosci. 2013;33:3240–50.
VonDran MW, Singh H, Honeywell JZ, Dreyfus CF. Levels of BDNF impact oligodendrocyte lineage cells following a cuprizone lesion. J Neurosci. 2011;31:14182–90.
Linker RA, Lee DH, Demir S, Wiese S, Kruse N, Siglienti I, et al. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133:2248–63.
Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, et al. Metformin improves health span and lifespan in mice. Nat Commun. 2013;4:2192.
Patil S, Jain P, Ghumatkar P, Tambe R, Sathaye S. Neuroprotective effect of metformin in MPTP-induced Parkinson’s disease in mice. Neurosci. 2014;277:747–54.
Ghadernezhad N, Khalaj L, Pazoki-Toroudi H, Mirmasoumi M, Ashabi G. Metformin pretreatment enhanced learning and memory in cerebral forebrain ischaemia: the role of the AMPK/BDNF/P70SK signalling pathway. Pharm Biol. 2016;54:2211–9.
Girard CA, Bemelmans P, Dufour N, et al. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924–33.
Mehrabi S, Sanadgol N, Barati M, Shahbazi A, Vahabzadeh G, Barzroudi M, et al. Evaluation of metformin effects in the chronic phase of spontaneous seizures in pilocarpine model of temporal lobe epilepsy. Metab Brain Dis. 2018;33:107–14.
Negrotto L, Farez MF, Correale J. Immunologic effects of metformin and pioglitazone treatment on metabolic syndrome and multiple sclerosis. JAMA neurology. 2016;73:520–8.
Fulmer CG, VonDran MW, Stillman AA, Huang Y, Hempstead BL, Dreyfus CF. Astrocyte-derived BDNF supports myelin protein synthesis after cuprizone-induced demyelination. J Neurosci. 2014;34:8186–96.
Lee JY, Petratos S. Multiple sclerosis: does Nogo play a role? Neuroscientist. 2013;19:394–408.
Yang Y, Liu Y, Wei P, Peng H, Winger R, Hussain RZ, et al. Silencing Nogo-A promotes functional recovery in demyelinating disease. Ann Neurol. 2010;67:498–507.
Hirokawa T, Zou Y, Kurihara Y, Jiang Z, Sakakibara Y, Ito H, et al. Regulation of axonal regeneration by the level of function of the endogenous Nogo receptor antagonist LOTUS. Sci Rep. 2017;7:12119.
Ineichen BV, Plattner PS, Good N, Martin R, Linnebank M, Schwab ME. Nogo-A antibodies for progressive multiple sclerosis. CNS Drugs. 2017;31:187–98.
Zemmar A, Chen CC, Weinmann O, et al. Oligodendrocyte-and neuron-specific Nogo-A restrict dendritic branching and spine density in the adult mouse motor cortex. Cereb Cortex. 2017;28:2109–17.
Ineichen BV, Kapitza S, Bleul C, Good N, Plattner PS, Seyedsadr MS, et al. Nogo-A antibodies enhance axonal repair and remyelination in neuro-inflammatory and demyelinating pathology. Acta Neuropathol. 2017;134:423–40.
Funding
This study was supported by the Shahrekord University of Medical Sciences, Shahrekord, Iran (Grant number: 1393-01-87-2325) and University of Zabol, Zabol, Iran (Grant number: 9618–5).
Author information
Authors and Affiliations
Contributions
Golab F. and Sanadgol N. conceived and designed this study. Houshmand F. and Barati M. analyzed data. Tanbakooie S. and Tabatabaei M. wrote the manuscript. All the authors contributed to conducting different experiments, read and approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The animal study was approved by the Animal Ethics Committee of the Shahrekord University of Medical Sciences, Shahrekord, Iran.
Consent for publication
All authors agree to publish our manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Houshmand, F., Barati, M., Golab, F. et al. Metformin-induced AMPK activation stimulates remyelination through induction of neurotrophic factors, downregulation of NogoA and recruitment of Olig2+ precursor cells in the cuprizone murine model of multiple sclerosis. DARU J Pharm Sci 27, 583–592 (2019). https://doi.org/10.1007/s40199-019-00286-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40199-019-00286-z