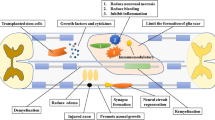
Abstract
Spinal cord injury (SCI) often leads to irreversible neuro-degenerative changes with life-long consequences. While there is still no effective therapy available, the results of past research have led to improved quality of life for patients suffering from partial or permanent paralysis. In this review we focus on the need, importance and the scientific value of experimental animal models simulating SCI in humans. Furthermore, we highlight modern imaging tools determining the location and extent of spinal cord damage and their contribution to early diagnosis and selection of appropriate treatment. Finally, we focus on available cellular and acellular therapies and novel combinatory approaches with exosomes and active biomaterials. Here we discuss the efficacy and limitations of adult mesenchymal stem cells which can be derived from bone marrow, adipose tissue or umbilical cord blood and its Wharton’s jelly. Special attention is paid to stem cell-derived exosomes and smart biomaterials due to their special properties as a delivery system for proteins, bioactive molecules or even genetic material.

Similar content being viewed by others
Abbreviations
- ATMSCs:
-
Adipose tissue mesenchymal stem cells
- ASIA score:
-
American Spinal Injury Association score
- bFGF:
-
Basic fibroblast growth factor
- BMSCs:
-
Bone marrow mesenchymal stem cells
- CM:
-
Conditioned media
- CNS:
-
Central nervous system
- DESI imaging:
-
Desorption electrospray ionization imaging technique
- DTI:
-
Diffusion tensor imaging
- DW-MRI:
-
Diffusion-weighted magnetic resonance imaging
- FDG:
-
Fluorodeoxyglucose
- GDNF:
-
Glial cell derived neurotrophic factor
- LC ACs:
-
Long-chain acylcarnitines
- lyso PC:
-
Lysophosphatidylcholines
- MALDI:
-
Matrix-assisted laser desorption/ionization
- MEPs:
-
Motor-evoked potentials
- MRI:
-
Magnetic resonance imaging
- MSC:
-
Mesenchymal stem cells
- NGF:
-
Nerve growth factor
- NT-3:
-
Neurotrophin-3 protein
- NT-4:
-
Neurotrophin-4 protein
- PAN/PVC:
-
Polyacrylonitrile/polyvinylchloride
- PGA:
-
Poly(glycolic acid)
- PET:
-
Positron emission tomography
- PHEMA:
-
Poly(2-hydroxyethyl methacrylate)
- PLA:
-
Poly(lactic acid)
- PLCL:
-
Poly(lactic-co-caprolactone)
- PLGA:
-
Poly(lactic-co-glycolic acid)
- PNS:
-
Peripheral nervous system
- PTFE:
-
Poly(tetrafluoro-ethylene)
- PVA:
-
Polyvinylalcohol
- ROS:
-
Reactive oxygen species
- SEPs:
-
Somato-sensory evoked potentials
- SCI:
-
Spinal cord injury
- TBI:
-
Traumatic brain injury
- UC:
-
Umbilical cord
- UCMSC:
-
Umbilical cord derived mesenchymal stem cells
- WJ:
-
Wharton’s jelly
- WJMSCs:
-
Wharton’s jelly derived mesenchymal stem cells
References
Dalamagkas K, Tsintou M, Seifalian AM (2018) Stem cells for spinal cord injuries bearing translational potential. Neural Regen Res 13:35–42. https://doi.org/10.4103/1673-5374.224360
Ellingson BM, Kurpad SN, Schmit BD (2008) Ex vivo diffusion tensor imaging and quantitative tractography of the rat spinal cord during long-term recovery from moderate spinal contusion. J Magn Reson Imaging 28:1068–1079. https://doi.org/10.1002/jmri.21578
Akhtar AZ, Pippin JJ, Sandusky CB (2008) Animal models in spinal cord injury: a review. Rev Neurosci 19:47–60
Nakae A, Nakai K, Yano K et al (2011) The animal model of spinal cord injury as an experimental pain model. J Biomed Biotechnol 2011:939023. https://doi.org/10.1155/2011/939023
Wrathall JR, Pettegrew RK, Harvey F (1985) Spinal cord contusion in the rat: production of graded, reproducible, injury groups. Exp Neurol 88:108–122
Rivlin AS, Tator CH (1978) Effect of duration of acute spinal cord compression in a new acute cord injury model in the rat. Surg Neurol 10:38–43
Blight AR (1991) Morphometric analysis of a model of spinal cord injury in guinea pigs, with behavioral evidence of delayed secondary pathology. J Neurol Sci 103:156–171
Lukovic D, Moreno-Manzano V, Lopez-Mocholi E et al (2015) Complete rat spinal cord transection as a faithful model of spinal cord injury for translational cell transplantation. Sci Rep 5:9640. https://doi.org/10.1038/srep09640
Sharif-Alhoseini M, Khormali M, Rezaei M et al (2017) Animal models of spinal cord injury: a systematic review. Spinal Cord 55:714–721. https://doi.org/10.1038/sc.2016.187
Kjell J, Olson L (2016) Rat models of spinal cord injury: from pathology to potential therapies. Dis Model Mech 9:1125–1137. https://doi.org/10.1242/dmm.025833
Cizkova D, Le Marrec-Croq F, Franck J et al (2014) Alterations of protein composition along the rostro-caudal axis after spinal cord injury: proteomic, in vitro and in vivo analyses. Front Cell Neurosci 8:105. https://doi.org/10.3389/fncel.2014.00105
Basso DM, Beattie MS, Bresnahan JC et al (1996) MASCIS evaluation of open field locomotor scores: effects of experience and teamwork on reliability. Multicenter Animal Spinal Cord Injury Study. J Neurotrauma 13:343–359. https://doi.org/10.1089/neu.1996.13.343
Navarro R, Juhas S, Keshavarzi S et al (2012) Chronic spinal compression model in minipigs: a systematic behavioral, qualitative, and quantitative neuropathological study. J Neurotrauma 29:499–513. https://doi.org/10.1089/neu.2011.2076
Noble LJ, Wrathall JR (1989) Correlative analyses of lesion development and functional status after graded spinal cord contusive injuries in the rat. Exp Neurol 103:34–40
Vanický I, Urdzíková L, Saganová K et al (2001) A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J Neurotrauma 18:1399–1407. https://doi.org/10.1089/08977150152725687
Jeffery ND, Smith PM, Lakatos A et al (2006) Clinical canine spinal cord injury provides an opportunity to examine the issues in translating laboratory techniques into practical therapy. Spinal Cord 44:584–593. https://doi.org/10.1038/sj.sc.3101912
Lim JH, Jung CS, Byeon YE et al (2007) Establishment of a canine spinal cord injury model induced by epidural balloon compression. J Vet Sci 8:89–94
Foditsch EE, Miclaus G, Patras I et al (2018) A new technique for minimal invasive complete spinal cord injury in minipigs. Acta Neurochir (Wien) 160:459–465. https://doi.org/10.1007/s00701-017-3442-3
Lee JHT, Jones CF, Okon EB et al (2013) A novel porcine model of traumatic thoracic spinal cord injury. J Neurotrauma 30:142–159. https://doi.org/10.1089/neu.2012.2386
Mills CD, Hains BC, Johnson KM, Hulsebosch CE (2001) Strain and model differences in behavioral outcomes after spinal cord injury in rat. J Neurotrauma 18:743–756. https://doi.org/10.1089/089771501316919111
Sedý J, Urdzíková L, Jendelová P, Syková E (2008) Methods for behavioral testing of spinal cord injured rats. Neurosci Biobehav Rev 32:550–580. https://doi.org/10.1016/j.neubiorev.2007.10.001
Chaovipoch P, Jelks KAB, Gerhold LM et al (2006) 17beta-estradiol is protective in spinal cord injury in post- and pre-menopausal rats. J Neurotrauma 23:830–852. https://doi.org/10.1089/neu.2006.23.830
Inamasu J, Nakamura Y, Ichikizaki K (2003) Induced hypothermia in experimental traumatic spinal cord injury: an update. J Neurol Sci 209:55–60
David BT, Steward O (2010) Deficits in bladder function following spinal cord injury vary depending on the level of the injury. Exp Neurol 226:128–135. https://doi.org/10.1016/j.expneurol.2010.08.014
Liebscher T, Schnell L, Schnell D et al (2005) Nogo-A antibody improves regeneration and locomotion of spinal cord-injured rats. Ann Neurol 58:706–719. https://doi.org/10.1002/ana.20627
Basso DM, Beattie MS, Bresnahan JC (1995) A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12:1–21. https://doi.org/10.1089/neu.1995.12.1
Tung A, Herrera S, Szafran MJ et al (2005) Effect of sleep deprivation on righting reflex in the rat is partially reversed by administration of adenosine A1 and A2 receptor antagonists. Anesthesiology 102:1158–1164
Ryken TC, Hadley MN, Walters BC et al (2013) Radiographic assessment. Neurosurgery 72(Suppl 2):54–72. https://doi.org/10.1227/NEU.0b013e318276edee
Janssen M, Nabih A, Moussa W et al (2011) Evaluation of diagnosis techniques used for spinal injury related back pain. Pain Res Treat. https://doi.org/10.1155/2011/478798doi: .
Gupta V (2013) Positron emission tomography in spinal cord disease. Mayo Clin Proc 88:1188–1190. https://doi.org/10.1016/j.mayocp.2013.09.004
Mori S, Zhang J (2006) Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539. https://doi.org/10.1016/j.neuron.2006.08.012
Parizel PM, van der Zijden T, Gaudino S et al (2010) Trauma of the spine and spinal cord: imaging strategies. Eur Spine J Off Publ Eur Spine Soc Eur Spinal Deform Soc Eur Sect Cerv Spine Res Soc 19(Suppl 1):S8–S17. https://doi.org/10.1007/s00586-009-1123-5
Vargas MI, Gariani J, Delattre BA et al (2015) Three-dimensional MR imaging of the brachial plexus. Semin Musculoskelet Radiol 19:137–148. https://doi.org/10.1055/s-0035-1546300
Quanico J, Hauberg-Lotte L, Devaux S et al (2018) 3D MALDI mass spectrometry imaging reveals specific localization of long-chain acylcarnitines within a 10-day time window of spinal cord injury. Sci Rep 8:16083. https://doi.org/10.1038/s41598-018-34518-0
Adams SH, Hoppel CL, Lok KH et al (2009) Plasma acylcarnitine profiles suggest incomplete long-chain fatty acid β-oxidation and altered tricarboxylic acid cycle activity in type 2 diabetic African-American women. J Nutr 139:1073–1081. https://doi.org/10.3945/jn.108.103754
Rutkowsky JM, Knotts TA, Ono-Moore KD et al (2014) Acylcarnitines activate proinflammatory signaling pathways. Am J Physiol Endocrinol Metab 306:E1378–E1387. https://doi.org/10.1152/ajpendo.00656.2013
Viader A, Sasaki Y, Kim S et al (2013) Aberrant Schwann cell lipid metabolism linked to mitochondrial deficits leads to axon degeneration and neuropathy. Neuron 77:886–898. https://doi.org/10.1016/j.neuron.2013.01.012
Devaux S, Cizkova D, Quanico J et al (2016) Proteomic analysis of the spatio-temporal based molecular kinetics of acute spinal cord injury identifies a time- and segment-specific window for effective tissue repair. Mol Cell Proteomics 15:2641–2670. https://doi.org/10.1074/mcp.M115.057794
Roux A, Muller L, Jackson SN et al (2016) Mass spectrometry imaging of rat brain lipid profile changes over time following traumatic brain injury. J Neurosci Methods 272:19–32. https://doi.org/10.1016/j.jneumeth.2016.02.004
Girod M, Shi Y, Cheng J-X, Cooks RG (2011) Mapping lipid alterations in traumatically injured rat spinal cord by desorption electrospray ionization imaging mass spectrometry. Anal Chem 83:207–215. https://doi.org/10.1021/ac102264z
Ahuja CS, Wilson JR, Nori S et al (2017) Traumatic spinal cord injury. Nat Rev Dis Primer 3:17018. https://doi.org/10.1038/nrdp.2017.18
Fawcett JW (2009) Recovery from spinal cord injury: regeneration, plasticity and rehabilitation. Brain 132:1417–1418. https://doi.org/10.1093/brain/awp121
Kubinová Š, Syková E (2009) Nanotechnology for treatment of stroke and spinal cord injury. Nanomedicine 5:99–108. https://doi.org/10.2217/nnm.09.93
Angeli CA, Boakye M, Morton RA et al (2018) Recovery of over-ground walking after chronic motor complete spinal cord injury. N Engl J Med 379:1244–1250. https://doi.org/10.1056/NEJMoa1803588
Gill ML, Grahn PJ, Calvert JS et al (2018) Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat Med 24:1677. https://doi.org/10.1038/s41591-018-0175-7
Musselman KE, Shah M, Zariffa J (2018) Rehabilitation technologies and interventions for individuals with spinal cord injury: translational potential of current trends. J Neuroeng Rehabil 15:40. https://doi.org/10.1186/s12984-018-0386-7
Sykova E, Forostyak S (2013) Stem cells in regenerative medicine. Laser Ther 22:87–92. https://doi.org/10.5978/islsm.13-RE-01
Kubinová Š, Syková E (2012) Biomaterials combined with cell therapy for treatment of spinal cord injury. Regen Med 7:207–224. https://doi.org/10.2217/rme.11.121
Grulova I, Slovinska L, Blaško J et al (2015) Delivery of alginate scaffold releasing two trophic factors for spinal cord injury repair. Sci Rep. https://doi.org/10.1038/srep13702
Syková E, Homola A, Mazanec R et al (2006) Autologous bone marrow transplantation in patients with subacute and chronic spinal cord injury. Cell Transplant 15:675–687
Syková E, Jendelová P, Urdzíková L et al (2006) Bone marrow stem cells and polymer hydrogels—two strategies for spinal cord injury repair. Cell Mol Neurobiol 26:1113–1129. https://doi.org/10.1007/s10571-006-9007-2
Nandoe Tewarie RDS, Nandoe RDS, Hurtado A et al (2006) Bone marrow stromal cells for repair of the spinal cord: towards clinical application. Cell Transplant 15:563–577
Cizkova D, Novotna I, Slovinska L et al (2011) Repetitive intrathecal catheter delivery of bone marrow mesenchymal stromal cells improves functional recovery in a rat model of contusive spinal cord injury. J Neurotrauma 28:1951–1961. https://doi.org/10.1089/neu.2010.1413
Cízková D, Rosocha J, Vanický I et al (2006) Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell Mol Neurobiol 26:1167–1180. https://doi.org/10.1007/s10571-006-9093-1
Osaka M, Honmou O, Murakami T et al (2010) Intravenous administration of mesenchymal stem cells derived from bone marrow after contusive spinal cord injury improves functional outcome. Brain Res 1343:226–235. https://doi.org/10.1016/j.brainres.2010.05.011
Forostyak S, Sykova E (2017) Neuroprotective potential of cell-based therapies in ALS: from bench to bedside. Front Neurosci. https://doi.org/10.3389/fnins.2017.00591
Vishnubalaji R, Al-Nbaheen M, Kadalmani B et al (2012) Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res 347:419–427. https://doi.org/10.1007/s00441-011-1306-3
Danisovic L, Varga I, Polák S et al (2009) Comparison of in vitro chondrogenic potential of human mesenchymal stem cells derived from bone marrow and adipose tissue. Gen Physiol Biophys 28:56–62
Elman JS, Li M, Wang F et al (2014) A comparison of adipose and bone marrow-derived mesenchymal stromal cell secreted factors in the treatment of systemic inflammation. J Inflamm Lond Engl 11:1. https://doi.org/10.1186/1476-9255-11-1
Ahmadian Kia N, Bahrami AR, Ebrahimi M et al (2011) Comparative analysis of chemokine receptor’s expression in mesenchymal stem cells derived from human bone marrow and adipose tissue. J Mol Neurosci MN 44:178–185. https://doi.org/10.1007/s12031-010-9446-6
Hsiao ST-F, Asgari A, Lokmic Z et al (2012) Comparative analysis of paracrine factor expression in human adult mesenchymal stem cells derived from bone marrow, adipose, and dermal tissue. Stem Cells Dev 21:2189–2203. https://doi.org/10.1089/scd.2011.0674
Huang JI, Kazmi N, Durbhakula MM et al (2005) Chondrogenic potential of progenitor cells derived from human bone marrow and adipose tissue: a patient-matched comparison. J Orthop Res Off Publ Orthop Res Soc 23:1383–1389. https://doi.org/10.1016/j.orthres.2005.03.008.1100230621
Balasubramanian S, Thej C, Venugopal P et al (2013) Higher propensity of Wharton’s jelly derived mesenchymal stromal cells towards neuronal lineage in comparison to those derived from adipose and bone marrow. Cell Biol Int 37:507–515. https://doi.org/10.1002/cbin.10056
Kim D-W, Staples M, Shinozuka K et al (2013) Wharton’s jelly-derived mesenchymal stem cells: phenotypic characterization and optimizing their therapeutic potential for clinical applications. Int J Mol Sci 14:11692–11712. https://doi.org/10.3390/ijms140611692
Zhou C, Yang B, Tian Y et al (2011) Immunomodulatory effect of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells on lymphocytes. Cell Immunol 272:33–38. https://doi.org/10.1016/j.cellimm.2011.09.010
Krupa P, Vackova I, Ruzicka J et al (2018) The effect of human mesenchymal stem cells derived from Wharton’s jelly in spinal cord injury treatment is dose-dependent and can be facilitated by repeated application. Int J Mol Sci 19:1503. https://doi.org/10.3390/ijms19051503
Riordan NH, Morales I, Fernández G et al (2018) Clinical feasibility of umbilical cord tissue-derived mesenchymal stem cells in the treatment of multiple sclerosis. J Transl Med 16:57. https://doi.org/10.1186/s12967-018-1433-7
Cantinieaux D, Quertainmont R, Blacher S et al (2013) Conditioned medium from bone marrow-derived mesenchymal stem cells improves recovery after spinal cord injury in rats: an original strategy to avoid cell transplantation. PLoS ONE 8:e69515. https://doi.org/10.1371/journal.pone.0069515
Kanekiyo K, Wakabayashi T, Nakano N et al (2018) Effects of intrathecal injection of the conditioned medium from bone marrow stromal cells on spinal cord injury in rats. J Neurotrauma 35:521–532. https://doi.org/10.1089/neu.2017.5201
Cizkova D, Cubinkova V, Smolek T et al (2018) Localized intrathecal delivery of mesenchymal stromal cells conditioned medium improves functional recovery in a rat model of spinal cord injury. Int J Mol Sci. https://doi.org/10.3390/ijms19030870
Asadi-Golshan R, Razban V, Mirzaei E et al (2018) Sensory and motor behavior evidences supporting the usefulness of conditioned medium from dental pulp-derived stem cells in spinal cord injury in rats. Asian Spine J 12:785–793. https://doi.org/10.31616/asj.2018.12.5.785
Théry C, Zitvogel L, Amigorena S (2002) Exosomes: composition, biogenesis and function. Nat Rev Immunol 2:569–579. https://doi.org/10.1038/nri855
Murgoci A-N, Cizkova D, Majerova P et al (2018) Brain-cortex microglia-derived exosomes: nanoparticles for glioma therapy. Chemphyschem Eur J Chem Phys Phys Chem 19:1205–1214. https://doi.org/10.1002/cphc.201701198
Lankford KL, Arroyo EJ, Nazimek K et al (2018) Intravenously delivered mesenchymal stem cell-derived exosomes target M2-type macrophages in the injured spinal cord. PLoS ONE 13:e0190358. https://doi.org/10.1371/journal.pone.0190358
Li D, Zhang P, Yao X et al (2018) Exosomes derived from miR-133b-modified mesenchymal stem cells promote recovery after spinal cord injury. Front Neurosci.https://doi.org/10.3389/fnins.2018.00845
Xu G, Ao R, Zhi Z et al (2018) miR-21 and miR-19b delivered by hMSC-derived EVs regulate the apoptosis and differentiation of neurons in patients with spinal cord injury. J Cell Physiol. https://doi.org/10.1002/jcp.27690
Straley KS, Foo CWP, Heilshorn SC (2010) Biomaterial design strategies for the treatment of spinal cord injuries. J Neurotrauma 27:1–19. https://doi.org/10.1089/neu.2009.0948
DeBrot A, Yao L (2018) The combination of induced pluripotent stem cells and bioscaffolds holds promise for spinal cord regeneration. Neural Regen Res 13:1677–1684. https://doi.org/10.4103/1673-5374.238602
Tsintou M, Dalamagkas K, Seifalian AM (2015) Advances in regenerative therapies for spinal cord injury: a biomaterials approach. Neural Regen Res 10:726–742. https://doi.org/10.4103/1673-5374.156966
Macaya D, Spector M (2012) Injectable hydrogel materials for spinal cord regeneration: a review. Biomed Mater Bristol Engl 7:012001. https://doi.org/10.1088/1748-6041/7/1/012001
Belkas JS, Munro CA, Shoichet MS et al (2005) Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials 26:1741–1749. https://doi.org/10.1016/j.biomaterials.2004.05.031
Cizkova D, Slovinska L, Grulova I et al (2015) The influence of sustained dual-factor presentation on the expansion and differentiation of neural progenitors in affinity-binding alginate scaffolds. J Tissue Eng Regen Med 9:918–929. https://doi.org/10.1002/term.1797
Trafton PG, Boyd CA (1984) Computed tomography of thoracic and lumbar spine injuries. J Trauma 24:506–515
Atesok K, Tanaka N, O’Brien A et al (2018) Posttraumatic spinal cord injury without radiographic abnormality. Adv Orthop 2018:7060654. https://doi.org/10.1155/2018/7060654
Guarnieri G, Izzo R, Muto M (2016) The role of emergency radiology in spinal trauma. Br J Radiol 89:20150833. https://doi.org/10.1259/bjr.20150833
Szwedowski D, Walecki J (2014) Spinal Cord Injury without Radiographic Abnormality (SCIWORA)—clinical and radiological aspects. Pol J Radiol 79:461–464. https://doi.org/10.12659/PJR.890944
Freund P, Curt A, Friston K, Thompson A (2013) Tracking changes following spinal cord injury: insights from neuroimaging. Neurosci Rev J Bringing Neurobiol Neurol Psychiatry 19:116–128. https://doi.org/10.1177/1073858412449192
Acknowledgements
This research was supported by: APVV 15-0613 (Dasa Cizkova), ERANET-AxonRepair (Dasa Cizkova), VEGA 2/0146/19, VEGA 1/0571/17, Grants from Ministère de L’Education Nationale, L’Enseignement Supérieur et de la Recherche, INSERM (Michel Salzet), SIRIC ONCOLille Grant INCD a-DGOS-Inserm 6041aa (Isabelle Fournier), IGA UVLF 06/2018 “Influence of Regeneration Capacity of Nervous Tissue in vitro through Adult Stem Cells products”.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Special Issue: In Honor of Prof. Eva Sykova.
Rights and permissions
About this article
Cite this article
Cizkova, D., Murgoci, AN., Cubinkova, V. et al. Spinal Cord Injury: Animal Models, Imaging Tools and the Treatment Strategies. Neurochem Res 45, 134–143 (2020). https://doi.org/10.1007/s11064-019-02800-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02800-w