Abstract
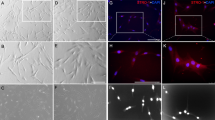
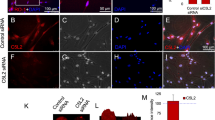
Dental pulp stem cells (DPSCs) were the most widely used seed cells in the field of neural regeneration and bone tissue engineering, due to their easily isolation, lack of ethical controversy, low immunogenicity and low rates of transplantation rejection. The purpose of this study was to investigate the role of basic fibroblast growth factor (bFGF) and nerve growth factor (NGF) on neural differentiation of DPSCs in vitro. DPSCs were cultured in neural differentiation medium containing NGF and bFGF alone or combination for 7 days. Then neural genes and protein markers were analyzed using western blot and RT-PCR. Our study revealed that bFGF and NGF increased neural differentiation of DPSCs synergistically, compared with bFGF and NGF alone. The levels of Nestin, MAP-2, βIII-tubulin and GFAP were the most highest in the DPSCs + bFGF + NGF group. Our results suggested that bFGF and NGF signifiantly up-regulated the levels of Sirt1. After treatment with Sirt1 inhibitor, western blot, RT-PCR and immunofluorescence staining showed that neural genes and protein markers had markedly decreased. Additionally, the ERK and AKT signaling pathway played a key role in the neural differentiation of DPSCs stimulated with bFGF + NGF. These results suggested that manipulation of the ERK and AKT signaling pathway may be associated with the differentiation of bFGF and NGF treated DPSCs. Our date provided theoretical basis for DPSCs to treat neurological diseases and repair neuronal damage.





Similar content being viewed by others
References
Liu Q, Cheng G, Wang Z, Zhan S, Xiong B, Zhao X (2015) Bone marrow-derived mesenchymal stem cells differentiate into nerve-like cells in vitro after transfection with brain-derived neurotrophic factor gene. In Vitr Cell Dev Biol Anim 51:319–327
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, DenBesten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci USA 97:13625–13630
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88:792–806
Pierdomenico L, Bonsi L, Calvitti M, Rondelli D, Arpinati M, Chirumbolo G, Becchetti E, Marchionni C, Alviano F, Fossati V, Staffolani N, Franchina M, Grossi A, Bagnara GP (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80:836–842
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Sakamoto K, Tauchi R, Wakao N, Imagama S, Hibi H, Kadomatsu K, Ishiguro N, Ueda M (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122:80–90
Hu Y, Zhang Y, Tian K, Xun C, Wang S, Lv D (2016) Effects of nerve growth factor and basic fibroblast growth factor dual gene modification on rat bone marrow mesenchymal stem cell differentiation into neuron-like cells in vitro. Mol Med Reports 13:49–58
van den Bos C, Mosca JD, Winkles J, Kerrigan L, Burgess WH, Marshak DR (1997) Human mesenchymal stem cells respond to fibroblast growth factors. Human cell 10:45–50
Tsutsumi S, Shimazu A, Miyazaki K, Pan H, Koike C, Yoshida E, Takagishi K, Kato Y (2001) Retention of multilineage differentiation potential of mesenchymal cells during proliferation in response to FGF. Biochem Biophys Res Commun 288:413–419
Ray J, Peterson DA, Schinstine M, Gage FH (1993) Proliferation, differentiation, and long-term culture of primary hippocampal neurons. Proc Natl Acad Sci USA 90:3602–3606
Colafrancesco V, Villoslada P (2011) Targeting NGF pathway for developing neuroprotective therapies for multiple sclerosis and other neurological diseases. Arch Ital Biol 149:183–192
Ding J, Cheng Y, Gao S, Chen J (2011) Effects of nerve growth factor and Noggin-modified bone marrow stromal cells on stroke in rats. J Neurosci Res 89:222–230
Karaoz E, Demircan PC, Saglam O, Aksoy A, Kaymaz F, Duruksu G (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol 136:455–473
Zhang J, Lu X, Feng G, Gu Z, Sun Y, Bao G, Xu G, Lu Y, Chen J, Xu L, Feng X, Cui Z (2016) Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: potential roles for spinal cord injury therapy. Cell Tissue Res 366(1):129–142. doi:10.1007/s00441-016-2402-1
Revollo JR, Li X (2013) The ways and means that fine tune Sirt1 activity. Trends Biochem Sci 38:160–167
Pillarisetti S (2008) A review of Sirt1 and Sirt1 modulators in cardiovascular and metabolic diseases. Recent Patents Cardiovasc Drug Discov 3:156–164
Kim YS, Lee YM, Park JS, Lee SK, Kim EC (2010) SIRT1 modulates high-mobility group box 1-induced osteoclastogenic cytokines in human periodontal ligament cells. J Cell Biochem 111:1310–1320
Lee SI, Park KH, Kim SJ, Kang YG, Lee YM, Kim EC (2012) Mechanical stress-activated immune response genes via Sirtuin 1 expression in human periodontal ligament cells. Clin Exp Immunol 168:113–124
Haigis MC, Guarente LP (2006) Mammalian sirtuins–emerging roles in physiology, aging, and calorie restriction. Genes Dev 20:2913–2921
Longo VD, Kennedy BK (2006) Sirtuins in aging and age-related disease. Cell 126:257–268
Donmez G, Outeiro TF (2013) SIRT1 and SIRT2: emerging targets in neurodegeneration. EMBO Mol Med 5:344–352
Herskovits AZ, Guarente L (2014) SIRT1 in neurodevelopment and brain senescence. Neuron 81:471–483
Li XH, Chen C, Tu Y, Sun HT, Zhao ML, Cheng SX, Qu Y, Zhang S (2013) Sirt1 promotes axonogenesis by deacetylation of Akt and inactivation of GSK3. Mol Neurobiol 48:490–499
Guo W, Qian L, Zhang J, Zhang W, Morrison A, Hayes P, Wilson S, Chen T, Zhao J (2011) Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J Neurosci Res 89:1723–1736
Sugino T, Maruyama M, Tanno M, Kuno A, Houkin K, Horio Y (2010) Protein deacetylase SIRT1 in the cytoplasm promotes nerve growth factor-induced neurite outgrowth in PC12 cells. FEBS Lett 584:2821–2826
Donmez G (2012) The neurobiology of sirtuins and their role in neurodegeneration. Trends Pharmacol Sci 33:494–501
Joe IS, Jeong SG, Cho GW (2015) Resveratrol-induced SIRT1 activation promotes neuronal differentiation of human bone marrow mesenchymal stem cells. Neurosci Lett 584:97–102
Feng X, Xing J, Feng G, Sang A, Shen B, Xu Y, Jiang J, Liu S, Tan W, Gu Z, Li L (2013) Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/beta-catenin signaling. Cell Mol Neurobiol 33:1023–1031
Feng X, Lu X, Huang D, Xing J, Feng G, Jin G, Yi X, Li L, Lu Y, Nie D, Chen X, Zhang L, Gu Z, Zhang X (2014) 3D porous chitosan scaffolds suit survival and neural differentiation of dental pulp stem cells. Cell Mol Neurobiol 34:859–870
Kim SU, de Vellis J (2009) Stem cell-based cell therapy in neurological diseases: a review. J Neurosci Res 87:2183–2200
Lee JH, Um S, Song IS, Kim HY, Seo BM (2014) Neurogenic differentiation of human dental stem cells in vitro. J Korean Assoc Oral Maxillofac Surg 40:173–180
Arthur A, Rychkov G, Shi S, Koblar SA, Gronthos S (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 26:1787–1795
de Almeida FM, Marques SA, Ramalho Bdos S, Rodrigues RF, Cadilhe DV, Furtado D, Kerkis I, Pereira LV, Rehen SK, Martinez AM (2011) Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J Neurotrauma 28:1939–1949
Fujino K, Ogura Y, Sato K, Nedachi T (2013) Potential neuroprotective effects of SIRT1 induced by glucose deprivation in PC12 cells. Neurosci Lett 557 Pt B:148–153
Bramanti V, Tomassoni D, Grasso S, Bronzi D, Napoli M, Campisi A, Li Volti G, Ientile R, Amenta F, Avola R (2012) Cholinergic precursors modulate the expression of heme oxigenase-1, p21 during astroglial cell proliferation and differentiation in culture. Neurochem Res 37:2795–2804
Grasso S, Bramanti V, Tomassoni D, Bronzi D, Malfa G, Traini E, Napoli M, Renis M, Amenta F, Avola R (2014) Effect of lipoic acid and alpha-glyceryl-phosphoryl-choline on astroglial cell proliferation and differentiation in primary culture. J Neurosci Res 92:86–94
Park YD, Kim YS, Jung YM, Lee SI, Lee YM, Bang JB, Kim EC (2012) Porphyromonas gingivalis lipopolysaccharide regulates interleukin (IL)-17 and IL-23 expression via SIRT1 modulation in human periodontal ligament cells. Cytokine 60:284–293
Franke TF (2007) Akt-interacting proteins: attractive opposites. focus on “Carboxy-terminal modulator protein induces Akt phosphorylation and activation, thereby enhancing antiapoptotic, glycogen synthetic, and glucose uptake pathways”. Am J Physiol Cell Physiol 293:C1768–C1770
Freyberg Z, Ferrando SJ, Javitch JA (2010) Roles of the Akt/GSK-3 and Wnt signaling pathways in schizophrenia and antipsychotic drug action. Am J Psychiatry 167:388–396
Namikawa K, Honma M, Abe K, Takeda M, Mansur K, Obata T, Miwa A, Okado H, Kiyama H (2000) Akt/protein kinase B prevents injury-induced motoneuron death and accelerates axonal regeneration. J Neurosci: Off J Soc Neurosci 20:2875–2886
Markus A, Zhong J, Snider WD (2002) Raf and akt mediate distinct aspects of sensory axon growth. Neuron 35:65–76
Chung J, Kubota H, Ozaki Y, Uda S, Kuroda S (2010) Timing-dependent actions of NGF required for cell differentiation. PLoS One 5:e9011
Bramanti V, Grasso S, Tibullo D, Giallongo C, Raciti G, Viola M, Avola R (2015) Modulation of extracellular signal-related kinase, cyclin D1, glial fibrillary acidic protein, and vimentin expression in estradiol-pretreated astrocyte cultures treated with competence and progression growth factors. J Neurosci Res 93:1378–1387
Leevers SJ, Marshall CJ (1992) Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J 11:569–574
Bramanti V, Grasso S, Tibullo D, Giallongo C, Pappa R, Brundo MV, Tomassoni D, Viola M, Amenta F, Avola R (2016) Neuroactive molecules and growth factors modulate cytoskeletal protein expression during astroglial cell proliferation and differentiation in culture. J Neurosci Res 94:90–98
Delcroix GJ, Curtis KM, Schiller PC, Montero-Menei CN (2010) EGF and bFGF pre-treatment enhances neural specification and the response to neuronal commitment of MIAMI cells. Differ Res Biol Divers 80:213–227
Lam HJ, Patel S, Wang A, Chu J, Li S (2010) In vitro regulation of neural differentiation and axon growth by growth factors and bioactive nanofibers. Tissue Eng Part A 16:2641–2648
Bronzi D, Bramanti V, Tomassoni D, Laureanti F, Grasso S, Li Volsi G, Avola R (2010) Neural markers expression in rat bone marrow mesenchymal stem cell cultures treated with neurosteroids. Neurochem Res 35:2154–2160
Acknowledgements
This work was supported by Natural Science Foundation of China Grant (No. 81500809, 81501076); Jiangsu Natural Science Foundation (BK2011385); “Top Six Types of Talents” Financial Assistance of Jiangsu Province Grant (Nos. 2013-WSN-076, 2013-WSN-048); Nantong Health Bureau Youth Foundation of China (WQ2015016); Graduate Student Innovation of Science and Technology Projects in Jiangsu Province and in Nantong University (NO.SJLX-0588; NO.SJLX-0588); Nantong Natural Science Foundation (NO.BK2014038).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Jinlong Zhang, Min Lian and Peipei Cao contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhang, J., Lian, M., Cao, P. et al. Effects of Nerve Growth Factor and Basic Fibroblast Growth Factor Promote Human Dental Pulp Stem Cells to Neural Differentiation. Neurochem Res 42, 1015–1025 (2017). https://doi.org/10.1007/s11064-016-2134-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-2134-3