Abstract
Bone marrow-derived mesenchymal stem cells can differentiate into a variety of adult cells. Brain-derived neurotrophic factor (BDNF) is briefly active during differentiation and induces mesenchymal stem cells to differentiate into nerve cells. In this study, we cloned human BDNF to generate a recombinant pcDNA3.1(−)-BDNF vector and transfected the vector into bone marrow-derived mesenchymal stem cells. We selected these cells with Geneticin-418 to obtain BDNF-BMSCs, which were induced with retinoic acid to obtain induced BDNF-BMSCs. The transfected cells displayed the typical morphology and surface antigen profile of fibroblasts and were observed to express clusters of differentiation 29, 44, and 90 (observed in matrix and stromal cells), but not clusters of differentiation 31, 34, and 45 (observed in red blood cells and endothelial cells), via flow cytometry. Enzyme-linked immunosorbent assays showed that transfected bone marrow-derived mesenchymal stem cells secreted more BDNF than non-transfected bone marrow-derived mesenchymal stem cells. Immunocytochemistry and real-time reverse transcription polymerase chain reaction analysis showed that non-induced BDNF-BMSCs maintained a higher proliferative capacity and expressed higher amounts of brain-derived neurotrophic factor, nestin, neuron-specific enolase, and glial fibrillary acid protein than non-transfected bone marrow-derived mesenchymal stem cells. An additional increase was observed in the induced BDNF-BMSCs compared to the non-induced BDNF-BMSCs. This expression profile is characteristic of neurocytes. Our data demonstrate that bone marrow-derived mesenchymal stem cells transfected with the BDNF gene can differentiate into nerve-like cells in vitro, which may enable the generation of sufficient quantities of nerve-like cells for treatment of neuronal diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The treatment of neurological diseases caused by irreversible neuronal cell damage, necrosis, or loss of neuronal cells is a global problem. In recent years, research into cell transplantation and gene therapy has provided a novel paradigm for addressing this problem. Bone-marrow mesenchymal stem cells (BMSCs) are multipotent stem cells (Kronenwett and Haas 2006) possessing plasticity (Grove et al. 2004) and can be induced to differentiate into a variety of adult cell lineages, including neurocytes (Qi et al. 2010), osteoblasts (Li et al. 2011), chondrocytes (Alves da Silva et al. 2011), and hepatocytes (Lange et al. 2006). Furthermore, BMSCs are easily isolated, with low rates of transplantation rejection. Thus, BMSCs have been deemed a resource for cell transplantation and an effective vector for gene therapy (Li et al. 2008). The primary limitation in the use of BMSCs is their low prevalence in bone marrow (only 0.1%) and their propensity to differentiate primarily into fibroblasts in vivo, with differentiation at very low rates into neurocytes and gliocytes. Thus, the therapeutic potential of BMSCs for treating neuronal conditions is currently severely limited.
Neurotrophic factors, especially brain-derived neurotrophic factor (BDNF), play an important role in the development, differentiation, survival, and damage-repair of neurocytes (Benraiss et al. 2001). It has been reported that BDNF, which plays a primary role in the maturation, proliferation, and differentiation of neuronal cells, can induce BMSCs to differentiate into nerve cells in vivo (Brazelton et al. 2000; Lin et al. 2010) and in vitro (Zhao et al. 2004).
However, as a macromolecule, BDNF has minimal ability to cross the blood–brain barrier and is only briefly active because of its short half-life in vivo. Previous studies have reported stable growth factor expression with high expression levels when a vector carrying the target gene is transfected into BMSCs (Lee et al. 2001), as the BDNF gene is characterized by continuous and stable expression and can maintain BDNF activity for an extended period of time.
In this study, we cultured BMSCs derived from guinea pigs in vitro and transfected the BDNF gene into the BMSCs by electroporation. We examined the characteristics of the transfected BMSCs. The aim of our experiments was to determine whether the BDNF gene could promote the nerve-like cell differentiation of BMSCs in vitro.
Materials and Methods
Human BDNF clone and eukaryotic expression vector construction.
RNA was extracted from human fetal brain tissue using Trizol reagent (Life Technologies, Carlsbad, CA). cDNA was synthesized by reverse transcription (Promega, Madison, WI). The cycling conditions for PCR were 94°C for 2 min; followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 60 s; and then 72°C for 5 min. BDNF primers, designed with Premier 5.0 software (PREMIER Biosoft, Palo Alto, CA), were added into a cDNA mixture for PCR amplification (Promega). The primers used were designed as follows (forward and reverse, respectively): 5′-GCGAATTCATGACCATCCTTTTCCTTACT-3′ and 5′-GCGGATCCTTATCTTC-CCTTTTAATGGTCAAT-3′. After the correct product sequence was verified, the pcDNA3.1(−) (Life Technologies) plasmid was used to generate the eukaryotic expression vector pcDNA3.1(−)-BDNF. The recombinant plasmids were transfected into HEK293 cells (ATCC, Rockville, MD), with BDNF expression determined by western blot (Bio-Rad Laboratories, Hercules, CA).
BMSC isolation and culture.
Five red-eyed white guinea pigs weighing 250–300 g were bought from the Animal Feeding Center of the Medical College of Jinan University, PRC. All experimental procedures were approved by the Laboratory Animal Management Committee of Guangdong Province. BMSC isolation and culturing were performed as previously reported (Wang et al. 2009). The medullary cavities of guinea pig femurs and tibias were flushed repeatedly with Dulbecco’s modified Eagle medium (DMEM; Gibco, Carlsbad, CA). The cells were rinsed with lymphocyte-separating medium (main ingredients: dextran and acid meglumine diatrizoate) and centrifuged at 1,000 rpm for 20 min. The pelleted cells were re-suspended in DMEM and re-centrifuged for an additional 10 min. The pellet (with the leukocytic cream removed) was centrifuged at 1,000 rpm, washed twice for 10 min, and cultured in DMEM containing 10% (v/v) fetal bovine serum (FBS; Sigma, St. Louis, MO) at 37°C in a humidified atmosphere containing 5% CO2. The medium was changed after 48 h, followed by removal of non-adherent cells. The cells were passaged at a ratio of 1:2 after achieving 80% confluence, with the third passage being used in all subsequent experiments.
BMSC identification by flow cytometry.
Passage 3 BMSCs were incubated with fluorescent-conjugated antibodies at 37°C for 1 h in the dark following two washes with phosphate-buffered saline (PBS, 1×). Cells were analyzed in a FACSCalibur flow cytometer using Paint-A-Gate software (Becton Dickinson, Franklin Lakes, NJ). The following antibodies were used: phycoerythrin (PE)-labeled rat anti-rat cluster of differentiation (CD) 29, CD31, CD34, CD44, CD45, and CD90 (Santa Cruz Biotechnology, Santa Cruz, CA).
BMSC transfection.
Passage 3 BMSCs were digested and centrifuged after adjustment of the density of the suspended cells to 2 × 106/mL. Next, 0.5 mL of the cell suspension and 20 μg of pcDNA3.1(−)-BDNF were added to a 4-mm electrotransformation pool (Bio-Rad Laboratories). Electroporation was performed at settings of 1050 Uf for capacitance and 260 V for voltage. The cells were transferred into six-well plates and cultured for 48 h after electroporation, followed by selection with 50–200 μg/mL Geneticin (G418) sulfate (Sigma) for 10–14 d, with a medium change every 2–3 d.
BDNF-BMSC induction and differentiation.
After removal of G418, the cells were cultured for an additional 5–7 d in DMEM containing 15% FBS. BMSCs that were transfected with the BDNF gene (BDNF-BMSCs) were induced by 0.5 μmol/L retinoic acid (RA; Sigma) for an additional 5 d.
BMSC and BDNF-BMSC proliferation-MTT assay.
The viability of the cells was measured using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT; Sigma) assay. At each time point after seeding, the cells were incubated with 5 g/L MTT for 4 h at 37°C. The formazan granules generated by the live cells were dissolved in 150 mL of dimethyl sulfoxide (DMSO), and the absorbance at 490 nm was monitored using a model 550 microplate reader (Bio-Rad Laboratories).
Cell growth.
As a direct cell growth assay, 5,000 cells were seeded per well in 24-well culture plates and counted daily by Trypan Blue exclusion dye staining (Sigma) for a 1-w period, and cell growth curves were plotted.
Cell analysis.
Cell cycle analysis was performed using propidium iodide staining. The cell number at different phases of the cell cycle was analyzed by a FACSCanto II flow cytometer (Becton Dickinson). The proliferative index (PI) was calculated according to a previously published formula (Wang et al. 2007)
Detection of BDNF in the culture supernatant by enzyme-linked immunosorbent assay (ELISA).
The BDNF concentration in the culture supernatant of all groups was determined by ELISA (R&D Systems, Minneapolis, MN). The culture supernatant was collected after the cells were cultured for 1 wk, 2 wk, 1 mo, and 2 mo. The concentration of FBS in the culture medium was decreased gradually from 15% to 10%, and then to 5%. Finally, Mesen Cult-XF medium (Gibco) was used to culture the cells before collection of the culture supernatant. All reagents and working standards were prepared following the manufacturer’s instructions. In brief, 100 μL of the supernatant for testing was added to 96-well plates, gently mixed, and incubated at 4°C overnight. Each well was aspirated and washed according to the manufacturer’s instruction, and 100 μL of the diluted biotinylated anti-guinea pig BDNF monoclonal antibody was added to each well. Subsequently, the plate was incubated for 2 h at room temperature. Aspiration and washing were repeated, and 100 μL of diluted streptavidin–HRP was added to each well and incubated for 2 h at room temperature. After 100 μL of TMB/E was added at room temperature for 15 min, the reaction was stopped by adding 50 μL of stop solution. The optical density (OD) in each well was determined within 30 min by using a microplate reader (Model 550, Bio-Rad Laboratories) at 450 nm.
Detection of BDNF, nestin, NSE, and GFAP by immunocytochemistry.
Immunocytochemistry was performed using the SABC method (Shi et al. 1988): the culture medium was removed from adherent cells, and the cells were then fixed in 4% paraformaldehyde, incubated in a mixture of 30% hydrogen peroxide and pure methanol, incubated in goat serum, and then incubated with the anti-BDNF, anti-nestin, anti-NSE, and anti-GFAP (1:300 dilution for all; Wuhan Boster, Wuhan, China) primary antibodies overnight in a 4°C refrigerator. The following day, incubation with the HRP-labeled rabbit anti-mouse secondary antibody (Wuhan Boster, Wuhan, China) was performed after rewarming of the samples, followed by staining with streptavidin-biotin complex (SABC; Wuhan Boster) and 3,3′-diaminobenzidine (DAB; Wuhan Boster) and counterstaining with hematoxylin. The samples were then dehydrated, coverslipped, and sealed. We selected ten non-overlapping visual fields in each well by inverted phase-contrast microscopy (Olympus, Tokyo, Japan); positively dyed and total cells were then counted.
Analysis of BDNF, GFAP, nestin, and NSE gene expression by real-time quantitative RT-PCR.
The expression of BDNF and neural lineage markers, including nestin, NSE, and GFAP, was analyzed in all groups by real-time RT-PCR. Cycling conditions were: 94°C for 2 min; followed by 30 cycles of 95°C for 30 s, 58°C for 30 s, and 72°C for 60 s; and finally 72°C for 5 min. The BDNF primers were described above; the other primers used were as follows (forward and reverse, respectively): for nestin, 5′-TCCAGAAACTCAAGCACCAC-3′ and 5′-CCACCGTATCTTCCCACCT-3′; for NSE, 5′-AACTGCCCCTGTATCGCCA-3′ and 5′-TTTTCCGTGTAGCCAGCCT-3′; and for GFAP, 5′-GCTTCCTGGAACAGCAAAAC-3′ and 5′-GGCTTCATCTGCTTCCTGTC-3′.
Statistical analysis.
The data are expressed as the mean ± standard deviation (\( \overline{x}\pm \mathrm{S}\mathrm{D} \)). Statistical analysis was performed using SPSS 15.0 statistical software (Chicago, IL). ANOVA was employed for comparison among groups. P < 0.05 was considered statistically significant.
Results
Construction of BDNF gene-containing eukaryotic expression vector.
The coding sequence of BDNF was generated by RT-PCR (Fig. 1A ). The eukaryotic expression vector pCDNA3.1(−)-BDNF was then successfully constructed. BDNF was confirmed to be present in HEK293 cells transfected with the pcDNA3.1(−)-BDNF plasmid. The size of the protein band conformed to that expected (Fig. 1B ).
Coding sequence (CDS) of the BDNF gene and expression of pcDNA3.1(−)-BDNF in HEK293 cells. (A) BDNF gene CDS. The CDS of the BDNF gene can be clearly observed (M: DL2000 DNA Marker, 1: BDNF CDS). (B) Expression of pcDNA3.1(−)-BDNF in HEK293 cells. BDNF was detected by western blot in HEK293 cells transfected with pcDNA3.1(−)-BDNF, but not in HEK293 cells transfected with pcDNA3.1(−) (CON: HEK293 cells transfected with pcDNA3.1(−), BDNF: HEK293 cells transfected with pcDNA3.1(−)-BDNF).
BMSC phenotype identification.
Flow cytometric analysis demonstrated that BMSCs at passage 3 expressed CD29, CD44, and CD90 (biomarkers of BMSCs) but not CD31, CD34, and CD45 (Fig. 2), indicating that the BMSCs were of high purity.
BMSC morphology and post-transfection change.
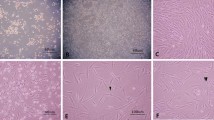
After 72 h of culture, the adherent cells were primarily characterized by their long spindle shape and large, single nucleus, similar to fibroblasts. However, spherical hematopoietic cells existed in suspension. Roughly 2 wk later, the adherent cells were overgrown in the flask bottom, with a monolayer that was mutually fused among cell colonies, and arranged with certain directionality (Fig. 3A ). The cellular morphology remained the same after passaging, although the rate of cellular proliferation gradually decreased with greater passage number. BMSC morphology and density did not change markedly after induction by RA (Fig. 3B ).
BMSC morphology and its change after transfection (magnification, ×100). (A) First generation of BMSCs. BMSCs cultured for 12 d were primarily characterized by long spindles and arranged with certain directivity. (B) RA-induced BMSCs. BMSC morphology and density did not markedly change after induction by RA. (C) Transfected BMSCs. Some BMSCs showed deformation after transfection with the human BDNF gene and treatment with G418, with a protruded soma and nerve-like form. (D) Induced BDNF-BMSCs. More BDNF-BMSCs differentiated and were deformed upon induction with retinoic acid (RA), where protruded soma were more apparent, and several cells were reticulated by connecting with one another.
Some BMSCs died after electroporation. After culture for 48 h, the viability of the transfected BMSCs was obviously higher than that of nontransfected BMSCs. Only cells transfected with BDNF survived G418 treatment. The transfection efficiency was approximately 28%. At 3–4 d after transfection, the cell density was still lower than that in non-transfected cells. Some surviving cells presented polygonal, fusiform, or ovoid shapes, along with cytoplasm retraction and concentration toward the nucleus together with a protruded soma (with the longer protruding end swelling into a cone shape) and a neuron-like form (Fig. 3C ). Subsequently, the number of amoeboid cells increased at a slower rate, with cells returning to normal growth rate under appropriate conditions at 5–7 d after G418 removal. The number of amoeboid BDNF-BMSCs increased further after RA addition, with apparent cellular protuberances, and in some cases, cellular networks (Fig. 3D ).
Proliferation analysis.
The following groups of BMSCs were analyzed: BMSCs that were neither BDNF-transfected nor RA-induced (non-induced BMSCs, a negative control group), BMSCs that were RA-induced (induced BMSCs), BDNF-BMSCs that were transfected but not RA-induced (non-induced BDNF-BMSCs), and BDNF-BMSCs that were RA-induced (induced BDNF-BMSCs).
The effect of the BDNF gene on BMSC proliferation in BMSCs and BDNF-BMSCs was determined using the MTT assay. At 48, 96, and 144 h post-seeding, there was no notable variation in viability between non-induced and induced BMSCs, whereas non-induced BDNF-BMSCs displayed a significant increase in viability compared with non-induced and induced BMSCs. An additional increase in viability was observed in induced BDNF-BMSCs compared to uninduced BDNF-BMSCs (P < 0.05; Fig. 4A ). Similar results were obtained for cell counts from days 2 to 7 (P < 0.05; Fig. 4B ). Moreover, cell cycle analysis showed that BDNF-BMSCs were present in apparently lower proportions in the G0/G1 phase and higher proportions in the S and G2/M phases compared to BMSCs (Fig. 4C , Table 1). The proliferative index (PI) of BDNF-BMSCs increased significantly compared with that of BMSCs (P < 0.05; Table 1).
Proliferation in BMSCs of BDNF-BMSCs. (A) Proliferation was determined by MTT assay at 48, 96, and 144 h. Values are presented as the mean ± SD for three independent experiments. (B) Growth curves. BDNF-BMSCs displayed a higher growth rate than BMSCs from days 2 to 7. The points represent the mean ± SD for triplicate experiments. (C) Representative histograms of flow cytometric analysis for cell cycle distribution. The proportion of BDNF-BMSCs in the S and G2/M phases was higher and that in the G0/G1 phases was lower than that in BMSCs. # P < 0.05, compared with BMSCs (induced or non-induced). *P < 0.05, compared with non-induced BDNF-BMSCs.
BDNF secretion.
BMSCs (induced or non-induced) secreted BDNF at 1 wk. BDNF secretion gradually decreased with time and was almost undetectable at 2 mo. There was an obvious increase in BDNF secretion from non-induced BDNF–BMSCs compared with BMSCs at each time point, with differences of 661-fold (1 wk), 1,317-fold (2 wk), 1,728-fold (1 mo), and 4,094-fold (2 mo) compared with the non-induced BMSCs. There was also an increase, although not significant, in the BDNF concentration in induced BDNF-BMSCs compared to non-induced BDNF–BMSCs. The maximum BDNF secretion of BDNF–BMSCs (induced or non-induced) was observed at 2 wk, although BDNF expression was still observed even at 2 mo (Table 2).
Immunocytochemistry.
Immunocytochemical analysis showed that non-induced BMSCs expressed low levels of BDNF, nestin, NSE, and GFAP (Fig. 5 A1–A4). Similarly, low levels of BDNF, nestin, NSE, and GFAP were detected in induced BMSCs (Fig. 5 B1–B4). By contrast, over 98% of non-induced BDNF-BMSCs (Fig. 5 C1) and induced BDNF-BMSCs (Fig. 5 D1) stained positively for BDNF. The number of cells positively co-stained for nestin, NSE, and GFAP was significantly higher in non-induced BDNF-BMSCs than in BMSCs (Fig. 5 C2–C4). Furthermore, a higher number of cells positively co-stained for nestin, NSE, and GFAP were detected in induced BDNF-BMSCs than in non-induced BDNF-BMSCs (Fig. 5 D2–D4).
Immunocytochemical analysis of BMSCs and BDNF-BMSCs (brown staining highlighted by arrows, magnification ×200). (A1–A4) Non-induced BMSCs expressed low levels of BDNF, nestin, NSE, and GFAP. (B1–B4) Low levels of BDNF, nestin, NSE, and GFAP were also detected in induced BMSCs. (C1–C4) The number of cells positively co-stained with BDNF, nestin, NSE, and GFAP in non-induced BDNF-BMSCs was significantly higher than that in BMSCs. Over 98% of non-induced BDNF-BMSCs were positively stained for BDNF. (D1–D4) Over 98% of induced BDNF-BMSCs were positively stained for BDNF. More cells positively co-stained for nestin, NSE, and GFAP were detected in induced BDNF-BMSCs than in non-induced BDNF-BMSCs. (E) Rates of positive immunostaining in BMSCs and BDNF-BMSCs, as determined by immunocytochemistry for BDNF, nestin, NSE, and GFAP (%; mean ± SD). # P < 0.01 compared with non-induced BMSCs and induced BMSCs; *P < 0.05 compared with non-induced BDNF-BMSCs.
BMSCs (induced or non-induced) expressed low levels of BDNF, nestin, NSE, and GFAP, whereas BDNF-BMSCs (induced or non-induced) expressed higher levels of BDNF, nestin, NSE, and GFAP (P < 0.01). Furthermore, more cells positively co-stained with nestin, NSE, and GFAP were detected in induced BDNF-BMSCs than in non-induced BDNF-BMSCs (P < 0.05) (Fig. 5E ).
mRNA expression.
To further confirm secretion and neuronal differentiation in BMSCs and BDNF-BMSCs, we analyzed the mRNA expression levels of BDNF and specific biomarkers of neuronal differentiation (i.e., nestin, NSE, and GFAP) by real-time quantitative RT-PCR. The results were consistent with the immunocytochemistry results. Compared with non-induced BMSCs, the mRNA expression levels of BDNF, nestin, NSE, and GFAP in non-induced BDNF-BMSCs were 8.66-, 2.67-, 6.22-, and 2.58-fold (P < 0.01) higher, respectively. Moreover, the levels of nestin, NSE, and GFAP mRNA in induced BDNF-BMSCs were 1.92-, 2.04-, and 2.11-fold (P < 0.05) higher, respectively, than those in non-induced BDNF-BMSCs (Fig. 6).
Discussion
BMSCs are derived from mesoblasts, adhere to the walls of tissue culture flasks, and possess a spindle morphology with fibroblast-forming colonies (Jiang et al. 2002). Our findings indicate that BMSCs do not express antigens observed on the surface of red blood cells and endothelial cells (e.g., CD31, CD34, and CD45), but express specific surface markers of matrix and stromal cells (e.g., CD29, CD44, and CD90) (Baddoo et al. 2003; Herzog et al. 2003). These findings agree with the accreditation standards of animal-origin BMSCs published by the International Society for Cell Therapy (Dominici et al. 2006). The particular mechanism of BMSC cross-germ-layer differentiation into neurons, which are derived from ectoblasts, remains unclear. Undifferentiated BMSCs express not only mesodermal but also endodermal and ectodermal genes. Therefore, BMSCs are capable of producing mesodermal derivatives and perhaps also ectodermal derivatives through transformation, which may be the basis for cross-germ-layer differentiation (Woodbury et al. 2002).
It has been confirmed that the amino acid sequences of mammalian BDNF (Zhang et al. 2006), TrkB, and the p75 receptor (Dobrzańska et al. 2002) are highly homologous and conserved with regard to tissue distribution. Human and guinea pigs are both mammals. Therefore, we transfected the BMSCs of guinea pigs with the human BDNF gene in the study, expecting that the human BDNF would have an effect on guinea pigs.
Under normal circumstances, BMSCs secrete low levels of BDNF, and few differentiate into neurocytes. This conclusion is supported by the observation that non-induced BMSCs were, en toto, only slightly positive by immunocytochemistry and had low levels of mRNA expression for BDNF, nestin, NSE, and GFAP in our experiments. The differentiation pathways of BMSCs are affected by a variety of factors, including differentiation signals, growth factor types in a given microenvironment, and the extracellular matrix (Deng et al. 2001; Seki 2003). The mechanism by which clinicians may induce BMSCs to differentiate into target cells has been the subject of intensive research in cellular transplantation and gene therapy.
In the present study, BMSCs were transfected with the BDNF gene. With continuous BDNF expression, the transfected BMSCs displayed significantly higher proliferative capacity, more BDNF secretion, and a higher proportion of differentiation into nerve-like cells than primary BMSCs. Differentiated BDNF-BMSCs possessed the morphological features of neuronal cells and expressed the neuronal cell-specific markers nestin, NSE, and GFAP. Nestin is a neuronal stem cell-specific marker protein, whereas NSE and GFAP are neuron- and astrocyte-specific markers, respectively.
The immunoreactivity and mRNA expression levels of nestin, NSE, and GFAP were higher in BDNF-BMSCs (induced or non-induced) than in BMSCs (induced or non-induced). Moreover, the immunoreactivity and mRNA expression level of NSE were greater than those of GFAP in BDNF-BMSCs (induced or non-induced), indicating that BDNF-BMSCs possessed characteristics of neuronal cells and suggesting that BDNF overexpression could enhance the differentiation of BMSCs into nerve-like cells, principally into neuron-like cells. These observations are consistent with previously reported studies (Jouhilahti et al. 2008). Although the mechanism underlying the BDNF-mediated enhancement of proliferation and differentiation remains unclear, it has been widely reported that BDNF binds to HNGFR TrkB or LNGFR P75NGFR on the membranes of BMSCs, activating tyrosine kinase (TK) and subsequently activating intracellular signaling pathways such as those involving mitogen-activated protein kinase (MAPK), calmodulin kinase (CaMK), and phosphatidylinositol-3-kinase (PI3K). This process causes BMSCs to proliferate more rapidly and differentiate from undifferentiated cells into mature neurons (Lu 2003; Tapia-Arancibia et al. 2004; Chai et al. 2007).
RA can induce cells to proliferate and differentiate by activating signal transduction upon binding to retinoic acid receptors (RARs) on the nuclear membrane, thereby affecting the transcriptional regulation of downstream genes and exerting biological effects (Nezzar et al. 2007). RA also enhances the expression of RARβ and the transcription factor islet-1/2, which is important for neural development. It is therefore used in neuronal induction media to promote the proliferation and differentiation of BMSCs into nerve-like cells (Bi et al. 2010). In our experiment, the immunoreactivity and mRNA expression levels of nestin, NSE, and GFAP in induced BDNF-BMSCs were higher than those in non-induced BDNF-BMSCs, consistent with the results of Bi et al. (2010). RA induces TrkB expression and switches on the TrkB-BDNF signal transduction pathway, enhancing the inductive effect of BDNF on cell differentiation (Kaplan et al. 1993). However, RA is a chemical reagent and thus has concentration-dependent effects. In particular, it has little effect on cells at low concentrations (for example, 0.5 μmol/L), whereas it has cytotoxic effects such as teratogenesis and apoptosis at high concentrations (Lu et al. 2009). To prevent toxicity, we treated the cells with a low concentration of RA (0.5 μmol/L). Thus, in this study, the RA did not have a marked effect on BMSCs. However, it evidently promoted the proliferation and differentiation of BDNF-BMSCs synergistically with BDNF.
Conclusions
In conclusion, we have confirmed that BDNF enhances the differentiation of BMSCs into nerve-like cells in vitro. We examined the characteristics of the differentiated nerve-like cells with regard to morphological features, BDNF secretion, and expression of neural cell markers. Some investigators have evaluated whether the differentiated cells possess the structural and functional characteristics of neuronal cells based on the ultrastructural and electrophysiological aspects of the cells, obtaining promising, although preliminary, results (Akiyama et al. 2002). The cellular differentiation mechanisms will be the focus of future research. The present findings may be useful for the treatment of nervous system diseases.
References
Akiyama Y, Radtke C, Kocsis JD (2002) Remyelination of the rat spinal cord transplantation of identified bone marrow stromal cells. J Neurosci 22(15):6623–6630
Alves da Silva ML, Martins A, Costa-Pinto AR, Correlo VM, Sol P, Bhattacharya M, Faria S, Reis RL, Neves NM (2011) Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J Tissue Eng Regen Med 5(9):722–732
Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, Phinney DG (2003) Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem 89(6):1235–1249
Benraiss A, Chmielnicki E, Lerner K, Roh D, Goldman SA (2001) Adenoviral brain-derived neurotrophic factor induces both neostriatal and olfactory neuronal recruitment from endogenous progenitor cell in the adult forebrain. J Neurosci 21(17):6718–6731
Bi Y, Gong M, Zhang X, Zhang X, Jiang W, Zhang Y, Chen J, Liu Y, He TC, Li T (2010) Pre-activation of retinoid signaling facilitates neuronal differentiation of mesenchymal stem cells. Dev Growth Differ 52(5):419–431
Brazelton TR, Rossi F, Keshet GI, Blau HM (2000) From marrow to brain: expression of neuronal phenotypes in adult mice. Science 290(5497):1775–1779
Chai LH, Wu SX, Yan WH, Ma YF (2007) Human bone marrow mesenchymal stem cells differentiated into dopaminergenic neurons in vitro. Sheng Wu Gong Cheng Xue Bao 23(2):252–256
Deng W, Obrocka M, Fischer I, Prockop DJ (2001) In vitro differentiation of human marrow stromal cells into early progenitors of neural cells by conditions that increase intracellular cyclic AMP. Biochem Biophys Res Commun 282(1):148–152
Dobrzańska Z, Wieckiewicz J, Bigda J (2002) Molecular cloning and sequencing of partial cDNA of tumor necrosis factor and p75 tumor necrosis factor receptor of Syrian golden hamster (Mesocricetus auratus) with the use of universal primers. Acta Biochim Pol 49(2):427–431
Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A, Prockop DJ, Horwitz E (2006) Minimal criteria for defining multipotent mesenchymal stem cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Grove JE, Bruscia E, Krause DS (2004) Plasticity of bone marrow-derived stem cells. Stem Cells 22(4):487–500
Herzog EL, Chai L, Krause DS (2003) Plasticity of marrow-derived stem cells. Blood 102(10):3483–3493
Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, Du J, Aldrich S, Lisberg A, Low WC, Largaespada DA, Verfaillie CM (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418(6893):41–49
Jouhilahti EM, Peltonen S, Peltonen J (2008) Class III beta-tubulin is a component of the mitotic spindle in multiple cell types. J Histochem Cytochem 56(12):1113–1119
Kaplan DR, Matsumoto K, Lucarelli E, Thiele CJ (1993) Induction of TrkB by retinoic acid mediates biologic responsiveness to BDNF and differentiation of human neuroblastoma cells. Neuron 11(2):321–331
Kronenwett R, Haas R (2006) Differentiation potential of stem cells from bone marrow. Med Klin (Munich) 101(Suppl 1):182–185, Article in German
Lange C, Bassler P, Lioznov MV, Bruns H, Kluth D, Zander AR, Fiegel HC (2006) Hepatocytic gene expression in cultured rat mesenchymal stem cells. Transplant Proc 37(1):276–279
Lee K, Majumdar MK, Buyaner D, Hendricks JK, Pittenger MF, Mosca JD (2001) Human mesenchymal stem cells maintain transgene expression during expansion and differentiation. Mol Ther 3(6):857–866
Li L, Li F, Qi H, Feng G, Yuan K, Deng H, Zhou H (2008) Coexpression of Pdx1 and betacellulin in mesenchymal stem cells could promote the differentiation of nestin-positive epithelium-like progenitors and pancreatic islet-like spheroids. Stem Cells Dev 17(4):815–823
Li Z, Wang Y, Wang XN, Lan AP, Wu W (2011) Strontium ranelate promotes osteogenic differentiation of rat bone marrow mesenchymal stem cells by increasing bone morphogenetic protein-7 expression. Nan Fang Yi Ke Da Xue Xue Bao 31(11):1949–1953, Article in Chinese
Lin J, Wang C, Wu Z (2010) Preliminary study on effects of human brain-derived neurotrophic factor gene-modified bone marrow mesenchymal stem cells by intravenous transplantation on structure and function of rat injured spinal cord. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 24(8):982–987, Article in Chinese
Lu B (2003) BDNF and activity-dependent synaptic modulation. Learn Mem 10(2):86–98
Lu J, Tan L, Li P, Gao H, Fang B, Ye S, Geng Z, Zheng P, Song H (2009) All-trans retinoic acid promotes neural lineage entry by pluripotent embryonic stem cells via multiple pathways. BMC Cell Biol 10:57
Nezzar H, Chiambaretta F, Marceau G, Blanchon L, Faye B, Dechelotte P, Rigal D, Sapin V (2007) Molecular and metabolic retinoid pathways in the human ocular surface. Mol Vis 13:1641–1650
Qi X, Shao M, Peng H, Bi Z, Su Z, Li H (2010) In vitro differentiation of bone marrow stromal cells into neurons and glial cells and differential protein expression in a two-compartment bone marrow stromal cell/neuron co-culture system. J Clin Neurosci 17(7):908–913
Seki T (2003) Microenvironmental elements supporting adult hippocampal neurogenesis. Anat Sci Int 78(2):69–78
Shi ZR, Itzkowitz SH, Kim YS (1988) A comparison of three immunoperoxidase techniques for antigen detect ion in colorectal carcinoma tissues. J Histochem Cytochem 36(3):317–322
Tapia-Arancibia L, Rage F, Givalois L, Arancibia S (2004) Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol 25(2):77–107
Wang FZ, Sha L, Zhang WY, Wu LY, Qiao L, Li N, Zhang XD, Ye LH (2007) Involvement of hepatitis B X-interacting protein (HBXIP) in proliferation regulation of cells. Acta Pharmacol Sin 28(3):431–438
Wang YJ, Sun ZR, Qiu XF, Li Y, Qin JZ, Han XD (2009) Roles of Wnt/beta-catenin signaling in epithelial differentiation of mesenchymal stem cells. Biochem Biophys Res Commun 390(4):1309–1314
Woodbury D, Reynolds K, Black IB (2002) Adult bone marrow stromal stem cells express germline, ectodermal, endodermal and mesodermal genes prior to neurogenesis. J Neurosci Res 69(6):908–917
Zhang ZM, Wang HJ, Liu ZL, Xiong GM (2006) Whole sequence analysis of brain-derived neurotrophic factor gene in Asiatic Black Bear through faecal sampling. Yi Chuan 28(6):665–671
Zhao LX, Zhang J, Cao F, Meng L, Wang DM, Li YH, Jiao WC, Zheng M, Xu XH, Pei XT (2004) Modification of the brain-derived neurotrophic factor gene: a portal to transform mesenchymal stem cells into advantageous engineering cells for neuroregeneration and neuroprotection. Exp Neurol 190(2):396–406
Acknowledgment
This study was supported by the Medical Scientific Research Foundation of Zhuhai, Guangdong, China [grant number 2011B040102031].
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: T. Okamoto
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0), which permits use, duplication, adaptation, distribution, and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Liu, Q., Cheng, G., Wang, Z. et al. Bone marrow-derived mesenchymal stem cells differentiate into nerve-like cells in vitro after transfection with brain-derived neurotrophic factor gene. In Vitro Cell.Dev.Biol.-Animal 51, 319–327 (2015). https://doi.org/10.1007/s11626-015-9875-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11626-015-9875-1