Abstract
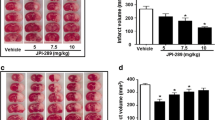
Olaparib was the first poly(ADP-ribose)polymerase inhibitor approved by Food and Drug Administration for oncology treatment. However, its neuroprotective effects have not been elucidated. This study aimed to evaluate the effects of olaparib in transient cerebral ischemia. A mouse model of transient middle cerebral artery occlusion was used. Reperfusion was performed at 2 h after ischemia. Different doses of olaparib (1, 3, 5, 10 and 25 mg/kg) were administered intraperitoneally immediately after reperfusion. Twenty-four hours after ischemia, the neurological score was assessed, and grip and string tests were performed to evaluate the behavioral deficits in the mice. Cresyl violet staining was used to assess cerebral edema and the lesion volume. Immunohistochemistry was performed to evaluate the expression of blood–brain barrier proteins collagen IV and claudin-5, as well as extravasation of IgG. Ischemia induced a neurological deficit, which was significantly ameliorated by olaparib at 3 and 5 mg/kg. However, this neuroprotective effect was not observed in mice treated with either low-dose or high-dose olaparib. Both 3 and 5 mg/kg olaparib markedly reduced cerebral infarction volume, but not cerebral edema. The expression of collagen IV decreased after cerebral ischemia, which was improved by olaparib at 3 and 5 mg/kg. These results were confirmed by the reduction of IgG extravasation with olaparib. Olaparib showed clear neuroprotective effects in transient ischemic mice mainly through the reduction of cerebral infarction and blood–brain barrier damage.





Similar content being viewed by others
References
Group IST, Sandercock P, Wardlaw JM et al (2012) The benefits and harms of intravenous thrombolysis with recombinant tissue plasminogen activator within 6 h of acute ischaemic stroke (the third international stroke trial [IST-3]): a randomised controlled trial. Lancet 379(9834):2352–2363
Lozano R, Naghavi M, Foreman K et al (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2095–2128
Murray CJ, Vos T, Lozano R et al (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2197–2223
Gerace E, Pellegrini-Giampietro DE, Moroni F, Mannaioni G (2015) Poly(ADP-ribose)polymerase 1 (PARP-1) activation and Ca(2 +) permeable alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) channels in post-ischemic brain damage: new therapeutic opportunities? CNS Neurol Disord: Drug Targets 14(5):636–646
Ishrat T, Sayeed I, Atif F, Hua F, Stein DG (2010) Progesterone and allopregnanolone attenuate blood-brain barrier dysfunction following permanent focal ischemia by regulating the expression of matrix metalloproteinases. Exp Neurol 226(1):183–190
Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G (2012) Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 379(9834):2364–2372
Majid A (2014) Neuroprotection in stroke: past, present, and future. ISRN Neurol 2014:515716
O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW (2006) 1,026 experimental treatments in acute stroke. Ann Neurol 59(3):467–477
Strosznajder R, Gadamski R, Walski M (2005) Inhibition of poly(ADP-ribose) polymerase activity protects hippocampal cells against morphological and ultrastructural alteration evoked by ischemia-reperfusion injury. Folia Neuropathol 43(3):156–165
Sodhi RK, Singh N, Jaggi AS (2010) Poly(ADP-ribose) polymerase-1 (PARP-1) and its therapeutic implications. Vascul Pharmacol 53(3–4):77–87
Moroni F (2008) Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr Opin Pharmacol 8(1):96–103
Fatokun AA, Dawson VL, Dawson TM (2014) Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol 171(8):2000–2016
Wang Y, Kim NS, Haince JF, Kang HC, David KK, Andrabi SA, Poirier GG, Dawson VL, Dawson TM (2011) Poly(ADP-ribose) (PAR) binding to apoptosis-inducing factor is critical for PAR polymerase-1-dependent cell death (parthanatos). Sci Signal 4(167):ra20
Eliasson MJ, Sampei K, Mandir AS, Hurn PD, Traystman RJ, Bao J, Pieper A, Wang ZQ, Dawson TM, Snyder SH, Dawson VL (1997) Poly(ADP-ribose) polymerase gene disruption renders mice resistant to cerebral ischemia. Nat Med 3(10):1089–1095
Endres M, Wang ZQ, Namura S, Waeber C, Moskowitz MA (1997) Ischemic brain injury is mediated by the activation of poly(ADP-ribose)polymerase. J Cereb Blood Flow Metab 17(11):1143–1151
Hendryk S, Czuba ZP, Jedrzejowska-Szypulka H, Szliszka E, Phillips VA, Threadgill MD, Krol W (2008) Influence of 5-aminoisoquinolin-1-one (5-AIQ) on neutrophil chemiluminescence in rats with transient and prolonged focal cerebral ischemia and after reperfusion. J Physiol Pharmacol 59(4):811–822
Kauppinen TM, Suh SW, Berman AE, Hamby AM, Swanson RA (2009) Inhibition of poly(ADP-ribose) polymerase suppresses inflammation and promotes recovery after ischemic injury. J Cereb Blood Flow Metab 29(4):820–829
Moroni F, Cozzi A, Chiarugi A, Formentini L, Camaioni E, Pellegrini-Giampietro DE, Chen Y, Liang S, Zaleska MM, Gonzales C, Wood A, Pellicciari R (2012) Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase. Br J Pharmacol 165(5):1487–1500
Teng F, Beray-Berthat V, Coqueran B, Lesbats C, Kuntz M, Palmier B, Garraud M, Bedfert C, Slane N, Bérézowski V, Szeremeta F, Hachani J, Scherman D, Plotkine M, Doan BT, Marchand-Leroux C, Margaill I (2013) Prevention of rt-PA induced blood-brain barrier component degradation by the poly(ADP-ribose)polymerase inhibitor PJ34 after ischemic stroke in mice. Exp Neurol 248:416–428
Bose CK, Basu N (2015) PARP inhibitors and more. J Turk Ger Gynecol Assoc 16(2):107–110
Lheureux S, Bowering V, Karakasis K, Oza AM (2015) Safety evaluation of olaparib for treating ovarian cancer. Expert Opin Drug Saf 14(8):1305–1316
Frechou M, Zhang S, Liere P, Delespierre B, Soyed N, Pianos A, Schumacher M, Mattern C, Guennoun R (2015) Intranasal delivery of progesterone after transient ischemic stroke decreases mortality and provides neuroprotection. Neuropharmacology 97:394–403
Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M, Zevenhoven J, Lau A, Boulter R, Cranston A, O’Connor MJ, Martin NM, Borst P, Jonkers J (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci USA 105(44):17079–17084
Riffell JL, Lord CJ, Ashworth A (2012) Tankyrase-targeted therapeutics: expanding opportunities in the PARP family. Nat Rev Drug Discov 11(12):923–936
Haddad M, Beray-Berthat V, Coqueran B, Palmier B, Szabo C, Plotkine M, Margaill I (2008) Reduction of hemorrhagic transformation by PJ34, a poly(ADP-ribose)polymerase inhibitor, after permanent focal cerebral ischemia in mice. Eur J Pharmacol 588(1):52–57
Wahl F, Allix M, Plotkine M, Boulu RG (1992) Neurological and behavioral outcomes of focal cerebral ischemia in rats. Stroke 23:267–272
Hall ED (1985) High-dose glucocorticoid treatment improves neurological recovery in head-injured mice. J Neurosurg 62:882–887
Paxinos G, Franklin KBJ (2001) The mouse brain in stereotaxic coordinates, 2nd edn. Academic Press, San Diego
El Amki M, Lerouet D, Coqueran B, Curis E, Orset C, Vivien D, Plotkine M, Marchand-Leroux C, Margaill I (2012) Experimental modeling of recombinant tissue plasminogen activator effects after ischemic stroke. Exp Neurol 238(2):138–144
Couturier JY, Ding-Zhou L, Croci N, Plotkine M, Margaill I (2003) 3-Aminobenzamide reduces brain infarction and neutrophil infiltration after transient focal cerebral ischemia in mice. Exp Neurol 184(2):973–980
Copin JC, Bengualid DJ, Da Silva RF, Kargiotis O, Schaller K, Gasche Y (2011) Recombinant tissue plasminogen activator induces blood-brain barrier breakdown by a matrix metalloproteinase-9-independent pathway after transient focal cerebral ischemia in mouse. Eur J Neurosci 34(7):1085–1092
Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Ramirez SH, Persidsky Y (2015) Poly(ADP-ribose) polymerase-1 inhibition in brain endothelium protects the blood-brain barrier under physiologic and neuroinflammatory conditions. J Cereb Blood Flow Metab 35(1):28–36
Iwashita A, Tojo N, Matsuura S, Yamazaki S, Kamijo K, Ishida J, Yamamoto H, Hattori K, Matsuoka N, Mutoh S (2004) A novel and potent poly(ADP-ribose) polymerase-1 inhibitor, FR247304 (5-chloro-2-[3-(4-phenyl-3,6-dihydro-1(2H)-pyridinyl)propyl]-4(3H)-quinazolinone), attenuates neuronal damage in in vitro and in vivo models of cerebral ischemia. J Pharmacol Exp Ther 310(2):425–436
Takahashi K, Pieper AA, Croul SE, Zhang J, Snyder SH, Greenberg JH (1999) Posttreatment with an inhibitor of poly(ADP-ribose) polymerase attenuates cerebral damage in focal ischemia. Brain Res 829:46–54
Yamamura E, Muto S, Yamada K, Sato Y, Iwase Y, Uno Y (2015) Chromosomal damage and micronucleus induction by MP-124, a novel poly(ADP-ribose) polymerase-1 (PARP-1) inhibitor: evidence for a non-DNA-reactive mode of action. Mutat Res, Genet Toxicol Environ Mutagen 782:1–8
Gerace E, Scartabelli T, Formentini L, Landucci E, Moroni F, Chiarugi A, Pellegrini-Giampietro DE (2012) Mild activation of poly(ADP-ribose) polymerase (PARP) is neuroprotective in rat hippocampal slice models of ischemic tolerance. Eur J Neurosci 36(1):1993–2005
Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O’Connor MJ, Ashworth A, Carmichael J, Kaye SB, Schellens JH, de Bono JS (2009) Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med 361(2):123–134
Javle M, Curtin NJ (2011) The role of PARP in DNA repair and its therapeutic exploitation. Br J Cancer 105(8):1114–1122
Fong PC, Yap TA, Boss DS, Carden CP, Mergui-Roelvink M, Gourley C, De Greve J, Lubinski J, Shanley S, Messiou C, A’Hern R, Tutt A, Ashworth A, Stone J, Carmichael J, Schellens JH, de Bono JS, Kaye SB (2009) Poly(ADP)-ribose polymerase inhibition: frequent durable responses in BRCA carrier ovarian cancer correlating with platinum free interval. J Clin Oncol 28:2512–2519
Forster MD, Dedes KJ, Sandhu S, Frentzas S, Kristeleit R, Ashworth A, Poole CJ, Weigelt B, Kaye SB, Molife LR (2011) Treatment with olaparib in a patient with PTEN-deficient endometrioid endometrial cancer. Nat Rev Clin Oncol 8:302–306
Acknowledgments
This investigation was supported by Grants from the National Natural Science Foundation of China (Project 81400961).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Additional information
Fei Teng and Ling Zhu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Teng, F., Zhu, L., Su, J. et al. Neuroprotective Effects of Poly(ADP-ribose)polymerase Inhibitor Olaparib in Transient Cerebral Ischemia. Neurochem Res 41, 1516–1526 (2016). https://doi.org/10.1007/s11064-016-1864-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-016-1864-6