Abstract
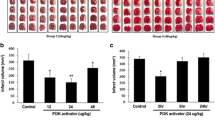
In patients with stroke and neurodegenerative diseases, overactivation of poly(ADP-ribose) polymerase-1 (PARP-1) causes harmful effects by inducing apoptosis, necrosis, neuroinflammation, and immune dysregulation. The current study investigated the neuroprotective effect of a novel PARP-1 inhibitor, JPI-289, in an animal model of ischemic stroke. A transient middle cerebral artery occlusion (tMCAO, 2 h) model was used to determine the therapeutic effect and the most effective dose and time window of administration of JPI-289. We also investigated the long-term outcomes of treatment with JPI-289 by diffusion-weighted imaging (DWI) and fluid-attenuated inversion recovery (FLAIR) MRI and by measuring neurological function at 24 h, 7 days, and 28 days after MCAO. The most effective dose and time window of administration of JPI-289 was 10 mg/kg administered 2 h after MCAO with reperfusion. Twenty-four hours after MCAO, infarct volume was reduced by 53% and the number of apoptotic cells was reduced by 56% compared with control. JPI-289 also reduced infarct volume by 16% in the permanent MCAO model. In an MRI-based study, initial infarct volume, as measured using DWI, was similar in the control and JPI-289-treated groups. However, infarct volume and brain swelling were significantly reduced in the group treated with JPI-289 (2 h) at 24 h and 7 days after MCAO. Neurological functions also improved in the group treated with JPI-289 (2 h) until 28 days after MCAO. Inhibition of PARP-1 has neuroprotective effects (reduction of infarct volume and brain swelling) in both tMCAO and pMCAO models of ischemic stroke.






Similar content being viewed by others
References
Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, Ezzati M, Shibuya K et al (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2197–2223. https://doi.org/10.1016/S0140-6736(12)61689-4
Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G (2012) Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 379(9834):2364–2372. https://doi.org/10.1016/S0140-6736(12)60738-7
Reis C, Akyol O, Ho WM, Araujo C, Huang L, Applegate Ii R, Zhang JH (2017) Phase I and phase II therapies for acute ischemic stroke: an update on currently studied drugs in clinical research. Biomed Res Int 2017(4863079):1–14. https://doi.org/10.1155/2017/4863079
Pena ID, Borlongan C, Shen G, Davis W (2017) Strategies to extend thrombolytic time window for ischemic stroke treatment: an unmet clinical need. J Stroke 19(1):50–60. https://doi.org/10.5853/jos.2016.01515
Savitz SI, Baron JC, Yenari MA, Sanossian N, Fisher M (2017) Reconsidering neuroprotection in the reperfusion era. Stroke 48(12):3413–3419. https://doi.org/10.1161/STROKEAHA.117.017283
Rouleau M, Patel A, Hendzel MJ, Kaufmann SH, Poirier GG (2010) PARP inhibition: PARP1 and beyond. Nat Rev Cancer 10(4):293–301. https://doi.org/10.1038/nrc2812
Strosznajder RP, Czubowicz K, Jesko H, Strosznajder JB (2010) Poly(ADP-ribose) metabolism in brain and its role in ischemia pathology. Mol Neurobiol 41(2-3):187–196. https://doi.org/10.1007/s12035-010-8124-6
Moroni F, Chiarugi A (2009) Post-ischemic brain damage: targeting PARP-1 within the ischemic neurovascular units as a realistic avenue to stroke treatment. FEBS J 276(1):36–45. https://doi.org/10.1111/j.1742-4658.2008.06768.x
Fatokun AA, Dawson VL, Dawson TM (2014) Parthanatos: mitochondrial-linked mechanisms and therapeutic opportunities. Br J Pharmacol 171(8):2000–2016. https://doi.org/10.1111/bph.12416
Kim Y, Kim YS, Noh MY, Lee H, Joe B, Kim HY, Kim J, Kim SH et al (2017) Neuroprotective effects of a novel poly(ADP-ribose) polymerase-1 inhibitor, JPI-289, in hypoxic rat cortical neurons. Clin Exp Pharmacol Physiol 44(6):671–679. https://doi.org/10.1111/1440-1681.12757
Laudisi F, Sambucci M, Pioli C (2011) Poly (ADP-ribose) polymerase-1 (PARP-1) as immune regulator. Endocr Metab Immune Disord Drug Targets 11(4):326–333. https://doi.org/10.2174/187153011797881184
Egi Y, Matsuura S, Maruyama T, Fujio M, Yuki S, Akira T (2011) Neuroprotective effects of a novel water-soluble poly(ADP-ribose) polymerase-1 inhibitor, MP-124, in in vitro and in vivo models of cerebral ischemia. Brain Res 1389:169–176. https://doi.org/10.1016/j.brainres.2011.03.031
Haddad M, Rhinn H, Bloquel C, Coqueran B, Szabo C, Plotkine M, Scherman D, Margaill I (2006) Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br J Pharmacol 149(1):23–30. https://doi.org/10.1038/sj.bjp.0706837
Koh SH, Park Y, Song CW, Kim JG, Kim K, Kim J, Kim MH, Lee SR et al (2004) The effect of PARP inhibitor on ischaemic cell death, its related inflammation and survival signals. Eur J Neurosci 20(6):1461–1472. https://doi.org/10.1111/j.1460-9568.2004.03632.x
Moroni F (2008) Poly(ADP-ribose)polymerase 1 (PARP-1) and postischemic brain damage. Curr Opin Pharmacol 8(1):96–103. https://doi.org/10.1016/j.coph.2007.10.005
Rom S, Zuluaga-Ramirez V, Dykstra H, Reichenbach NL, Ramirez SH, Persidsky Y (2015) Poly(ADP-ribose) polymerase-1 inhibition in brain endothelium protects the blood-brain barrier under physiologic and neuroinflammatory conditions. J Cereb Blood Flow Metab 35(1):28–36. https://doi.org/10.1038/jcbfm.2014.167
Takahashi K, Pieper AA, Croul SE, Zhang J, Snyder SH, Greenberg JH (1999) Post-treatment with an inhibitor of poly(ADP-ribose) polymerase attenuates cerebral damage in focal ischemia. Brain Res 829(1-2):46–54. https://doi.org/10.1016/S0006-8993(99)01335-9
Teng F, Beray-Berthat V, Coqueran B, Lesbats C, Kuntz M, Palmier B, Garraud M, Bedfert C et al (2013) Prevention of rt-PA induced blood-brain barrier component degradation by the poly(ADP-ribose)polymerase inhibitor PJ34 after ischemic stroke in mice. Exp Neurol 248:416–428. https://doi.org/10.1016/j.expneurol.2013.07.007
Teng F, Zhu L, Su J, Zhang X, Li N, Nie Z, Jin L (2016) Neuroprotective effects of poly(ADP-ribose)polymerase inhibitor olaparib in transient cerebral ischemia. Neurochem Res 41(7):1516–1526. https://doi.org/10.1007/s11064-016-1864-6
Koh SH, Yoo AR, Chang DI, Hwang SJ, Kim SH (2008) Inhibition of GSK-3 reduces infarct volume and improves neurobehavioral functions. Biochem Biophys Res Commun 371(4):894–899. https://doi.org/10.1016/j.bbrc.2008.05.006
Kim YS, Yoo A, Son JW, Kim HY, Lee YJ, Hwang S, Lee KY, Lee YJ et al (2017) Early activation of phosphatidylinositol 3-kinase after ischemic stroke reduces infarct volume and improves long-term behavior. Mol Neurobiol 54(7):5375–5384. https://doi.org/10.1007/s12035-016-0063-4
Chen TY, Goyagi T, Toung TJ, Kirsch JR, Hurn PD, Koehler RC, Bhardwaj A (2004) Prolonged opportunity for ischemic neuroprotection with selective kappa-opioid receptor agonist in rats. Stroke 35(5):1180–1185. https://doi.org/10.1161/01.STR.0000125011.93188.c6
Zhao X, Liu SJ, Zhang J, Strong R, Aronowski J, Grotta JC (2005) Combining insulin-like growth factor derivatives plus caffeinol produces robust neuroprotection after stroke in rats. Stroke 36(1):129–134. https://doi.org/10.1161/01.STR.0000149624.87661.18
Chen J, Li Y, Wang L, Zhang Z, Lu D, Lu M, Chopp M (2001) Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke 32(4):1005–1011. https://doi.org/10.1161/01.STR.32.4.1005
Sughrue ME, Mocco J, Komotar RJ, Mehra A, D’Ambrosio AL, Grobelny BT, Penn DL, Connolly ES Jr (2006) An improved test of neurological dysfunction following transient focal cerebral ischemia in rats. J Neurosci Methods 151(2):83–89. https://doi.org/10.1016/j.jneumeth.2005.04.023
Zhang RL, Chopp M, Zhang ZG, Jiang Q, Ewing JR (1997) A rat model of focal embolic cerebral ischemia. Brain Res 766(1-2):83–92. https://doi.org/10.1016/S0006-8993(97)00580-5
Haddad M, Beray-Berthat V, Coqueran B, Plotkine M, Marchand-Leroux C, Margaill I (2013) Combined therapy with PJ34, a poly(ADP-ribose)polymerase inhibitor, reduces tissue plasminogen activator-induced hemorrhagic transformations in cerebral ischemia in mice. Fundam Clin Pharmacol 27(4):393–401. https://doi.org/10.1111/j.1472-8206.2012.01036.x
Zhang L, Zhang ZG, Zhang RL, Lu M, Adams J, Elliott PJ, Chopp M (2001) Postischemic (6-hour) treatment with recombinant human tissue plasminogen activator and proteasome inhibitor PS-519 reduces infarction in a rat model of embolic focal cerebral ischemia. Stroke 32(12):2926–2931. https://doi.org/10.1161/hs1201.100207
Schreiber V, Dantzer F, Ame JC, de Murcia G (2006) Poly(ADP-ribose): novel functions for an old molecule. Nat Rev Mol Cell Biol 7(7):517–528. https://doi.org/10.1038/nrm1963
Moroni F, Cozzi A, Chiarugi A, Formentini L, Camaioni E, Pellegrini-Giampietro DE, Chen Y, Liang S et al (2012) Long-lasting neuroprotection and neurological improvement in stroke models with new, potent and brain permeable inhibitors of poly(ADP-ribose) polymerase. Br J Pharmacol 165(5):1487–1500. https://doi.org/10.1111/j.1476-5381.2011.01666.x
Patel MR, Bhatt A, Steffen JD, Chergui A, Murai J, Pommier Y, Pascal JM, Trombetta LD et al (2014) Discovery and structure-activity relationship of novel 2,3-dihydrobenzofuran-7-carboxamide and 2,3-dihydrobenzofuran-3(2H)-one-7-carboxamide derivatives as poly(ADP-ribose)polymerase-1 inhibitors. J Med Chem 57(13):5579–5601. https://doi.org/10.1021/jm5002502
Yao H, Ji M, Zhu Z, Zhou J, Cao R, Chen X, Xu B (2015) Discovery of 1-substituted benzyl-quinazoline-2,4(1H,3H)-dione derivatives as novel poly(ADP-ribose)polymerase-1 inhibitors. Bioorg Med Chem 23(4):681–693. https://doi.org/10.1016/j.bmc.2014.12.071
Rosado MM, Bennici E, Novelli F, Pioli C (2013) Beyond DNA repair, the immunological role of PARP-1 and its siblings. Immunology 139(4):428–437. https://doi.org/10.1111/imm.12099
Chen S, Wu H, Klebe D, Hong Y, Zhang J, Tang J (2013) Regulatory T cell in stroke: a new paradigm for immune regulation. Clin Dev Immunol 2013(689827):1–9. https://doi.org/10.1155/2013/689827
O’Collins VE, Macleod MR, Donnan GA, Horky LL, van der Worp BH, Howells DW (2006) 1,026 experimental treatments in acute stroke. Ann Neurol 59(3):467–477. https://doi.org/10.1002/ana.20741
Fisher M, Feuerstein G, Howells DW, Hurn PD, Kent TA, Savitz SI, Lo EH, Group, S (2009) Update of the stroke therapy academic industry roundtable preclinical recommendations. Stroke 40(6):2244–2250. https://doi.org/10.1161/STROKEAHA.108.541128
Liu F, Lang J, Li J, Benashski SE, Siegel M, Xu Y, McCullough LD (2011) Sex differences in the response to poly(ADP-ribose) polymerase-1 deletion and caspase inhibition after stroke. Stroke 42(4):1090–1096. https://doi.org/10.1161/STROKEAHA.110.594861
Yuan M, Siegel C, Zeng Z, Li J, Liu F, McCullough LD (2009) Sex differences in the response to activation of the poly (ADP-ribose) polymerase pathway after experimental stroke. Exp Neurol 217(1):210–218. https://doi.org/10.1016/j.expneurol.2009.02.012
Funding
This study was funded by grant of the Korea Drug Development Fund (KDDF-201410-08) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (A100453) for the clinical development of JPI-289.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
All national and institutional guidelines for the care and use of animals were followed.
Rights and permissions
About this article
Cite this article
Kim, Y., Kim, Y.S., Kim, H.Y. et al. Early Treatment with Poly(ADP-Ribose) Polymerase-1 Inhibitor (JPI-289) Reduces Infarct Volume and Improves Long-Term Behavior in an Animal Model of Ischemic Stroke. Mol Neurobiol 55, 7153–7163 (2018). https://doi.org/10.1007/s12035-018-0910-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-018-0910-6