Abstract
Purpose
Brain tumors, particularly glioblastoma multiforme (GBM), present significant prognostic challenges despite multimodal therapies, including surgical resection, chemotherapy, and radiotherapy. One major obstacle is the limited drug delivery across the blood–brain barrier (BBB). Focused ultrasound (FUS) combined with systemically administered microbubbles has emerged as a non-invasive, targeted, and reversible approach to transiently open the BBB, thus enhancing drug delivery. This review examines clinical trials employing BBB opening techniques to optimise pharmacotherapy for brain tumors, evaluates current challenges, and proposes directions for further research.
Methods
A systematic literature search was conducted in PubMed and ClinicalTrials.gov up to November 2023, searching for “ultrasound” AND “brain tumor”. The search yielded 1446 results. After screening by title and abstract, followed by full-text screening (n = 48), 35 studies were included in the analysis.
Results
Our analysis includes data from 11 published studies and 24 ongoing trials. The predominant focus of these studies is on glioma, including GMB and astrocytoma. One paper investigated brain metastasis from breast cancer. Evidence indicates that FUS facilitates BBB opening and enhances drug uptake following sonication. Exploration of FUS in the pediatric population is limited, with no published studies and only three ongoing trials dedicated to this demographic.
Conclusion
FUS is a promising strategy to safely disrupt the BBB, enabling precise and non-invasive lesion targeting, and enhance drug delivery. However, pharmacokinetic studies are required to quantitatively assess improvements in drug uptake. Most studies are phase I clinical trials, and long-term follow-up investigating patient outcomes is essential to evaluate the clinical benefit of this treatment approach. Further studies involving diverse populations and pathologies will be beneficial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain tumors continue to exhibit a poor prognosis, with less than 20% of patients surviving beyond 5 years post-diagnosis [1]. Glioblastoma multiforme (GBM) represents the most prevalent primary malignant brain tumor. Despite various therapeutic modalities, including surgery, chemotherapy and radiotherapy, substantial improvements in patient survival have not been realised [2]. A major contributing factor is the challenge of pharmacotherapies penetrating the blood–brain barrier (BBB) to reach the tumor at therapeutic concentrations [3]. Consequently, there is growing interest in research focused on safe and reversible BBB opening (BBBO), which holds promise for enhancing the delivery and efficacy of therapeutic agents [4].
The BBB, formed by microvascular endothelial cells that regulate molecule and ion transfer from the blood into the brain parenchyma, enables homeostasis and normal neuronal functioning [3]. These cells interact with astrocytes and pericytes to uphold the barrier’s integrity [5]. The challenge of drug delivery is compounded by localized vascular changes in tumors, which can increase interstitial fluid pressure (IFP), complicating the dynamics of drug delivery [6].
Several techniques are used for transient BBBO. One involves administering hyperosmotic agents like mannitol via intra-arterial infusion [7]. However, the dilutional effects of collateral arterial system in the Circle of Willis complicates its reproducibility [8]. Another method is convection-enhanced delivery (CED), which relies on the principles of bulk flow and uses stereotactic catheter to administer therapeutics directly into the target. However, backflow presents as a significant challenge, where the infusion penetrates through the catheter tract rather than reaching the targeted area [9]. This results in a dilutional effect at the tumor site, as the drug therapy advances to unintended areas. Other techniques, such as implanting drug-releasing polymers, and conjugation of pharmacotherapies to proteins, also face drawbacks (e.g. reduced delivery and rapid clearance from circulation) [10].
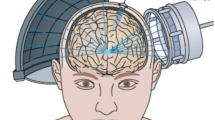
The use of focused ultrasound (FUS) to transiently open the BBB is under increasing research. This technique involves directing low-frequency ultrasound waves at targeted brain regions, producing microbubble-seeded acoustic cavitation and intravascular shear stress that can produce reversible permeability changes in the BBB (Fig. 1). The disruption allows therapeutic agents, such as chemotherapeutics or gene therapies, to penetrate brain tissue more effectively. FUS-mediated BBBO is non-invasive and can be precisely controlled, making it a promising approach for treating brain tumors and other neurological disorders while minimizing systemic side effects [11]. Currently, the use of ultrasound with microbubbles is the only non-invasive, targeted, and reversible method for transient BBBO to enhance drug delivery [12].
Schematic of FUS-Induced BBB Disruption. The application of ultrasound waves to targeted brain region induces microbubble-seeded acoustic cavitation. This process generates intravascular shear stress, leading to reversible changes in BBB permeability, thereby facilitating the enhanced penetration of therapeutic agents into brain tissue. Adapted from “Lipid-Based Microbubbles (MBs) as Ultrasound-Based Drug Delivery System” by BioRender.com (2024). Retrieved from https://app.biorender.com/biorender-templates
Microbubbles are micron-sized, gas-filled particles that are widely used as contrast agents in diagnostic ultrasound imaging. When coupled with therapeutic ultrasound, they can enhance targeted drug delivery by locally amplifying intravascular stresses. Low-frequency, low-intensity FUS causes microbubbles to oscillate in response to the alternating phases of the acoustic waves, causing temporary BBB disruption [6, 12]. Drugs can be administered either concurrently with microbubbles or bound to their shell via ligands for localized release [13].
Pre-clinical studies on mice, rats, rabbits, canines, and non-human primates (NHPs) have demonstrated safe, effective, and reversible BBBO with ultrasound and microbubbles, leading to human clinical trials to assess efficacy and safety in clinical settings [14,15,16,17,18,19]. For example, evidence has shown that FUS-mediated BBBO is safe in mice with diffuse intrinsic pontine gliomas (DIPG) [20,21,22]. Other studies have shown that BBBO does not affect cognitive performance of NHPs post-treatment, further indicating the safety of FUS [17, 23].
This review investigates current clinical trials on FUS-guided BBBO during pharmacotherapy administration for brain tumors, offering a comprehensive analysis of the current clinical landscape of FUS use in neuro-oncology. We critically assess existing challenges surrounding this treatment method and propose directions for further research.
Methods
The systematic literature search was conducted in PubMed and ClinicalTrials.gov from inception to 1st November 2023, searching for a combination of “ultrasound” AND “brain tumor”. The exact search terms used were [(focused ultrasound) OR (unfocused ultrasound) OR (pulsed ultrasound) OR (microbubble*)) AND ((glioma) OR (glioblastoma) OR (astrocytoma) OR (ependymoma) OR (medulloblastoma) OR (brain tumour*) OR (brain tumor*) OR (brain neoplasm*)].
Our literature search yielded 1446 results (Fig. 2). Two investigators (HZ and CA) independently determined eligibility of study after screening by title and abstract. Discrepancies were discussed and resolved through discussion with a therapeutic ultrasound (ANP) or pharmacy (MGB) expert. Studies were then screened by full text (n = 47), adhering to inclusion and exclusion criteria:
Inclusion criteria
Included studies were published in English involving participants with brain tumors, investigated the use of ultrasound to open the BBB, and reported relevant outcomes as primary or secondary endpoints.
Exclusion criteria
Excluded studies were not relevant to brain tumor treatment, lacked ultrasound intervention, or used FUS for thermal ablation or sonobiopsy but not BBBO for drug delivery. Additionally, we excluded animal and in vitro studies, as well as non-original work, e.g. reviews, comments, editorials, letters, and opinion articles.
Results
Published studies
After screening, data was collected from 11 publications across 6 centers involving 7 clinical trials and 61 patients in total. The majority of studies were Phase 0 or Phase I trials evaluating the safety and feasibility of BBBO through FUS treatment (Table 1).
Glioma, particularly recurrent GBM, is most frequently investigated for FUS treatment. Other types of gliomas investigated include astrocytoma, oligodendroglioma, and diffuse infiltrating glioma. One study investigated brain metastasis from human epidermal growth factor receptor 2 (HER-2) positive breast cancer [24]. Notably, all studies focused on adult patients, with a mean age of 50.7 years, and none of the published studies has thus far explored the pediatric population.
The drugs used following FUS-guided BBBO included paclitaxel [25] and carboplatin [26,27,28] for recurrent GBM, and temozolomide (TMZ) for GBM [29, 30] and astrocytoma [31]. Trastuzumab was used for HER-2 positive breast cancer brain metastasis [24].
Trials utilized three ultrasound systems, including the SonoCloud (CarThera, France), ExAblate Neuro (InSightec, Israel), and NaviFUS (Taiwan). SonoCloud system is an ultrasound device implanted during craniotomy for tumor removal. This allows for repeated BBBO over multiple chemotherapy cycles. ExAblate Neuro is an MR-guided hemispherical multi-element array, which has electronic steering capabilities and high targeting precision. The NaviFUS system is neuronavigation-guided and can be used in an outpatient setting outside the MRI.
Few studies reported evidence suggestive of enhanced drug uptake post-sonication. In one study the mean parenchymal paclitaxel concentration increased by 3.7-fold (from 0.037 to 0.139 μM) in treated patients, and carboplatin by 5.9-fold (from 0.991 to 5.878 μM) [25]. Another study showed a 35% average contrast enhancement, and chemotherapy concentration enhancements of 47 and 671% post-sonication in two patients, respectively [31].
MRI demonstrated FUS-induced BBBO, evident from discrete contrast extravasation on gadolinium-enhanced MRI immediately post-treatment [24, 25, 31]. The contrast extravasation occurred in a grid pattern with ExAblate which resolved within 24 h [24, 31], and in a cylindrical pattern with SonoCloud-9, which resolved within an hour [25]. Immediate side effects included transient headache, pin-site tenderness, and neurological deficits associated with sonicated regions, including weakness, dysarthria, and dysphasia. Side effects generally diminish with steroid treatment [29], and resolved within 1 to 48 h in one study [27]. In a phase I trial, patients receiving 260 mg/m2 of albumin-bound paclitaxel experienced grade 2 and 3 encephalopathy with low-intensity pulsed ultrasound and concomitant administration of intravenous microbubbles (LIPU-MB) [25]. This dose-limiting toxicity resolved, and treatment was recommenced at lower doses of 175 and 215 mg/m2, respectively. Additionally, neutropenia, leukopenia, and hypertension commonly manifested as grade 3–4 treatment-emergent adverse events [25].
Long-term patient outcomes in phase 0/I trials are often limited due to short follow-up periods. Those studies predominantly focus on safety and feasibility, as well as determining maximum safe dosage of drugs. Consequently, many studies have yet to report long-term outcomes, with follow-ups ranging from 1 to 15 months, and some omitting results entirely.
Ongoing trials
There are 24 ongoing trials currently investigating the use of FUS in neuro-oncology (Table 2). Similar to the published trials, the majority are in their early phases, with only 2 in phase III. 12 ongoing trials utilised the ExAblate device, 6 used SonoCloud, and 6 used neuronavigation-guided transducers (NaviFUS and UltraNav systems).
A variety of drugs, including carboplatin, doxorubicin, bevacizumab, are investigated. Each of these drugs has distinct molecular properties, such as molecular weight or lipophilicity, enabling them to readily pass through the BBB with the assistance of FUS-mediated BBBO, as shown in pre-clinical trials [35,36,37]. For example, the molecular mass of carboplatin is 371 Da, whereas bevacizumab has a mass of 149 kDa (Table 2). This variation in molecular mass results in different drug delivery enhancement when using FUS, even with identical treatment parameters.
Additionally, there are differences in the treatment pathway. Most studies focus on enhanced drug delivery following BBBO. Another pathway is to use FUS to open the BBB to mark the regions of infiltrating gliomas, in order to improve visualization during surgical resection and maximize total tumor resection. Currently, one ongoing trial (NCT04667715) is evaluating this endpoint, pointing the direction for further research.
Discussion
Pharmacotherapies
A range of medications in combination with FUS treatments are under investigation. TMZ, the first-line therapy of high-grade gliomas, exhibits high oral bioavailability and the ability to cross the BBB due to its lipophilicity and small size. Despite its efficacy, its cerebrospinal fluid concentration is only about 20% of plasma concentration [38], and the median survival in GBM patients following traditional treatment with surgery, radiation, and TMZ is only 14.6 months [38]. This limitation may stem from efflux by the P-glycoprotein 1 (P-gp), a common multidrug resistant protein abundant in BBB within cancerous tissues, as shown in rats [39]. Nonetheless, P-gp is shown to be down-regulated after treatment with FUS and microbubbles [40], and TMZ concentration has been shown to increase by 7.7-fold when BBBO is performed concomitantly [29]. The promising result following FUS, coupled with its inherent potency, renders it an ideal candidate for FUS trials. Notably, two published trials have already examined the effect of this drug with FUS [31, 34].
Albumin-bound paclitaxel is another medication that showed promising effects after treatment with LIPU-MB. As a chemotherapeutic agent, paclitaxel is 1400 times more potent than TMZ [25]. However, despite its potency, paclitaxel does not cross the BBB [41], and has not shown efficacy for glioma in clinical trials [42].
Devices
Magnetic resonance-guided focused ultrasound (MRgFUS) offers a promising approach, as multiple studies have demonstrated its ability to temporarily disrupt the BBB without damaging surrounding tissues [43]. MRgFUS delivers ultrasound energy with intraoperative imaging guidance and real-time feedback, enabling non-invasive, selective targeting of intracranial lesions, including those in deep and functionally critical regions [30].
Implant-based approaches for BBBO, such as the implantable SonoCloud device by CarThera, are beneficial as they can be implanted immediately following surgical removal of tumor, thereby avoiding the need for additional procedures. However, they are constrained by the direction of the transducer and have limited ability to precisely control the direction of BBBO (Table 3). In contrast, MRgFUS offers greater flexibility in selecting the target location and size, as the direction of ultrasound can be adjusted. Their ease of use and lack of need for targeting in each session make these transducers attractive for regular treatments in the same region.
MRgFUS disrupts the BBB through multiple mechanisms, such as direct disruption of tight junctions and induced transcytosis [44]. Intraoperative MRI enables the identification of bioeffects caused by BBB disruption, potentially reducing the risk of false-negative outcomes compared to implant-based methods. MRgFUS can also target any brain region with minimal tissue reflection at the tissue-skull boundary, especially when the stereotaxic frame is appropriately positioned. Furthermore, real-time acoustic feedback and power modulation facilitate precise control and adaptation of the BBBO magnitude and distribution, enhancing safety and efficacy [30].
However, MRgFUS procedures require fixation of stereotaxic frame with regular frame adjustments, which may cause discomfort and emotional stress [30]. The time and cost of MRI also needs to be considered. Additionally, as enhanced T1-weighted MRI is the gold standard for BBBO confirmation, gadolinium contrast needs to be administered, and as such, patients with poor renal function are often excluded.
Other methods that monitor microbubble activity such as passive acoustic detection and acoustic mapping could be used for predicting the outcome of FUS treatments and degree of drug delivery enhancement. However, these techniques have their own limitations, such as variable sensitivity, limited resolution, and computational speeds [45,46,47]. Imaging microbubble acoustic emissions in 2D and 3D can identify the spatial location of microbubble activity, which can be correlated with the degree of gadolinium penetration into the brain, a typical surrogate for BBBO confirmation, or directly with the degree of drug delivery [48]. All devices incorporate cavitation monitoring as a feedback mechanism, apart from CarThera.
Trial variability
The number of participants in ongoing trials is often limited, ranging from 3 to 57. The recently initiated SONOBIRD study, with around 560 participants enrolled across the globe (NCT05902169), will provide invaluable information on treatment response in a large cohort. Small sample sizes have limited the generalizability of trials, affecting the establishment of formalized standards for evaluating drug choice, device type, and treatment parameters. Variations exist in acoustic pressure/intensity, pulse length, center frequency, pulse repetition frequency, and total treatment time. These differences make it challenging to interpret the effects of ultrasound parameters, especially given the limited data on drug uptake. There appears to be a positive correlation between the number of cycles and duration of treatment with a higher incidence of side effects in some studies [28, 37]. More comparative studies are needed to evaluate the exact correlation due to the limited data available. Other parameters such as ethnicity, comorbidities, age, and grade of tumor, all interplay into the prognosis and suitability of each treatment.
Variability also exists in microbubble parameters among studies. The two microbubbles used across published trials are Definity (perflutren lipid microspheres, 4 or 10 µL/kg) and SonoVue (sulfur hexafluoride, 0.1 mL/kg, max 4.8 mL). A study in rats suggest similar BBBO effects under equivalent concentrations [49]. Future research should aim to standardize microbubble usage and dosing protocols to better monitor concentration effects in patients.
Safety
Appropriate ultrasound parameters are crucial to avoid risks such as erythrocyte extravasations in cerebral microvasculature, limiting the incremental ultrasound level below 0.8 mechanical index (MI) [50]. MRI abnormalities following FUS treatment include T2* hyperintensities within 24 h post-treatment, indicating brain edema, and susceptibility-weighted imaging hypointensities, indicating localized microhemorrhage [24].
Potential improvements to clinical trials
Only a few studies have reported quantitative data regarding change in drug concentrations post-FUS [25, 31]. Moreover, information on the restoration rate of BBB integrity is not generally provided, with limited exceptions, showing restoration within a few hours after procedure with SonoCloud [25]. Additionally, parameters such as pulse length, intensity, and pulse repetition frequency should be standardized to enable better comparisons of outcomes across different studies.
Furthermore, additional investigation is needed to assess the feasibility and specific considerations for treatment across diverse populations. No published studies have evaluated treatment feasibility in pediatrics, though there are ongoing trials for diffuse midline glioma (DMG) patients. DMG, also known as DIPG, is a rare brain tumor that primarily occurs in children between 2 and 9 years of age, with a poor prognosis and an average survival of 9–12 months after diagnosis [51]. DMG is well protected from circulating drugs due to intact BBB. Additionally, surgical resection is in general not feasible, due to its location within the brainstem and neighboring eloquent areas. These characteristics render FUS a promising therapeutic solution for DMG. Currently, there are three ongoing trials using FUS to enhance the delivery of etoposide, panobinostat, and doxorubicin for DMG, respectively, with additional studies in the planning stages. Moreover, pediatric patients require careful assessment due to anatomical variances and different neurodevelopmental stages. Common tumor types also differ, with medulloblastoma being the most prevalent. Trials targeting prominent pediatric tumor types are essential for advancing FUS applications.
Future directions
Currently, most published studies are in initial stages with small sample sizes. The poor prognosis of brain tumors complicates long-term follow-up for assessing the efficacy of FUS. Many trials focus on short-term safety, with follow-up periods often less than two years, as longer follow-up times are often ambitious given the disease course of brain tumors. Long-term patient outcomes are necessary to establish the validity and efficacy of the approach, which has the potential to inform future treatment guidelines and clinical practice. Additional trial data, coupled with molecular imaging techniques, will provide more defined understanding of the relationship between FUS dose, drug pharmacokinetics, and tumor response [24].
FUS holds promise beyond brain tumor treatment, with applications in other brain pathologies. In Alzheimer's disease, FUS-mediated BBB disruption is shown to reduce beta-amyloid and tau pathology [52]. Its feasibility has also been explored in amyotrophic lateral sclerosis (ALS) [53]. FUS-mediated BBBO may open avenues for otherwise incurable conditions, and further research is required to fully explore these possibilities.
Further exploration is needed in developing new small- or large-molecule pharmacotherapies for GBM, with various trials currently ongoing. In a placebo-controlled phase III trial, cediranib, an oral pan-vascular endothelial growth factor (VEGF) receptor tyrosine kinase inhibitor, did not improve progression-free survival in patients with recurrent GBM [54]. Despite this, it may benefit from concomitant FUS-mediated BBBO to improve clinical efficacy. The same applies to other drugs like tivozanib and sunitinib [55, 56]. Furthermore, promising in vitro chemotherapeutic agents should undergo investigation with FUS [57]. Additionally, targeted immunotherapies, such as monoclonal antibodies or CAR-T cell therapies, could benefit from localized and reversible FUS-mediated BBBO in brain tumor patients [58].
Conclusion
This systematic review summarized the published and ongoing clinical trials using FUS for targeted BBBO in brain tumors. Our findings indicate that FUS-mediated BBBO is a safe procedure with the potential to improve clinical outcomes. We also discussed challenges and areas for further study. Future research should aim to develop standardized, evidence-based protocols for drug and device choices, and treatment parameters for both adult and pediatric patients. Various device types and personalized pharmacotherapies should also be explored. Beyond the scope of brain tumors, FUS may benefit other conditions once its advantages and device accessibility are established.
Data availability
No datasets were generated or analysed during the current study.
References
Wen PY, Weller M, Lee EQ et al (2020) Glioblastoma in adults: a society for neuro-oncology (SNO) and European society of neuro-oncology (EANO) consensus review on current management and future directions. Neuro Oncol 22:1073–1113. https://doi.org/10.1093/neuonc/noaa106
Kumari S, Gupta R, Ambasta RK, Kumar P (2023) Multiple therapeutic approaches of glioblastoma multiforme: from terminal to therapy. Biochimica et Biophysica Acta (BBA) Rev Cancer 1878:188913. https://doi.org/10.1016/j.bbcan.2023.188913
Wu D, Chen Q, Chen X et al (2023) The blood–brain barrier: structure, regulation, and drug delivery. Signal Transduct Target Ther 8:217. https://doi.org/10.1038/s41392-023-01481-w
Kadry H, Noorani B, Cucullo L (2020) A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 17:69. https://doi.org/10.1186/s12987-020-00230-3
Abbott NJ (2005) Dynamics of CNS barriers: evolution, differentiation, and modulation. Cell Mol Neurobiol 25:5–23. https://doi.org/10.1007/s10571-004-1374-y
Sprowls SA, Arsiwala TA, Bumgarner JR et al (2019) Improving CNS delivery to brain metastases by blood-tumor barrier disruption. Trends Cancer 5:495–505. https://doi.org/10.1016/j.trecan.2019.06.003
Cosolo WC, Martinello P, Louis WJ, Christophidis N (1989) Blood–brain barrier disruption using mannitol: time course and electron microscopy studies. Am J Physiol 256:R443–R447. https://doi.org/10.1152/ajpregu.1989.256.2.R443
Linville RM, DeStefano JG, Sklar MB et al (2020) Modeling hyperosmotic blood–brain barrier opening within human tissue-engineered in vitro brain microvessels. J Cereb Blood Flow Metab 40:1517–1532. https://doi.org/10.1177/0271678X19867980
Stine CA, Munson JM (2019) Convection-enhanced delivery: connection to and impact of interstitial fluid flow. Front Oncol. https://doi.org/10.3389/fonc.2019.00966
Madsen SJ, Hirschberg H (2010) Site-specific opening of the blood–brain barrier. J Biophotonics 3:356–367. https://doi.org/10.1002/jbio.200900095
McDannold N, Zhang Y, Vykhodtseva N (2011) Blood–brain barrier disruption and vascular damage induced by ultrasound bursts combined with microbubbles can be influenced by choice of anesthesia protocol. Ultrasound Med Biol 37:1259–1270. https://doi.org/10.1016/j.ultrasmedbio.2011.04.019
Abe K, Taira T (2017) Focused ultrasound treatment, present and future. Neurol Med Chir (Tokyo) 57:386–391. https://doi.org/10.2176/nmc.ra.2017-0024
Tsutsui JM, Xie F, Porter RT (2004) The use of microbubbles to target drug delivery. Cardiovasc Ultrasound 2:23. https://doi.org/10.1186/1476-7120-2-23
Kobus T, Vykhodtseva N, Pilatou M et al (2016) Safety validation of repeated blood–brain barrier disruption using focused ultrasound. Ultrasound Med Biol 42:481–492. https://doi.org/10.1016/j.ultrasmedbio.2015.10.009
Blackmore DG, Turpin F, Mohamed AZ et al (2018) Multimodal analysis of aged wild-type mice exposed to repeated scanning ultrasound treatments demonstrates long-term safety. Theranostics 8:6233–6247. https://doi.org/10.7150/thno.27941
Olumolade OO, Wang S, Samiotaki G, Konofagou EE (2016) Longitudinal motor and behavioral assessment of blood–brain barrier opening with transcranial focused ultrasound. Ultrasound Med Biol 42:2270–2282. https://doi.org/10.1016/j.ultrasmedbio.2016.05.004
Downs ME, Buch A, Sierra C et al (2015) Long-term safety of repeated blood–brain barrier opening via focused ultrasound with microbubbles in non-human primates performing a cognitive task. PLoS ONE 10:e0125911. https://doi.org/10.1371/journal.pone.0125911
McDannold N, Arvanitis CD, Vykhodtseva N, Livingstone MS (2012) Temporary disruption of the blood–brain barrier by use of ultrasound and microbubbles: safety and efficacy evaluation in rhesus macaques. Cancer Res 72:3652–3663. https://doi.org/10.1158/0008-5472.CAN-12-0128
O’Reilly MA, Jones RM, Barrett E et al (2017) Investigation of the safety of focused ultrasound-induced blood–brain barrier opening in a natural canine model of aging. Theranostics 7:3573–3584. https://doi.org/10.7150/thno.20621
Englander ZK, Wei H-J, Pouliopoulos AN et al (2021) Focused ultrasound mediated blood–brain barrier opening is safe and feasible in a murine pontine glioma model. Sci Rep 11:6521. https://doi.org/10.1038/s41598-021-85180-y
Martinez P, Nault G, Steiner J et al (2023) MRI-guided focused ultrasound blood–brain barrier opening increases drug delivery and efficacy in a diffuse midline glioma mouse model. Neurooncol Adv. https://doi.org/10.1093/noajnl/vdad111
Ishida J, Alli S, Bondoc A et al (2021) MRI-guided focused ultrasound enhances drug delivery in experimental diffuse intrinsic pontine glioma. J Control Release 330:1034–1045. https://doi.org/10.1016/j.jconrel.2020.11.010
Pouliopoulos AN, Kwon N, Jensen G et al (2021) Safety evaluation of a clinical focused ultrasound system for neuronavigation guided blood–brain barrier opening in non-human primates. Sci Rep 11:15043. https://doi.org/10.1038/s41598-021-94188-3
Meng Y, Reilly RM, Pezo RC et al (2021) MR-guided focused ultrasound enhances delivery of trastuzumab to Her2-positive brain metastases
Sonabend AM, Gould AB, Amidei C et al (2023) Repeated blood–brain barrier opening with an implantable ultrasound device for delivery of albumin-bound paclitaxel in patients with recurrent glioblastoma: a phase 1 trial
Carpentier A, Canney M, Vignot A et al (2016) Clinical trial of blood–brain barrier disruption by pulsed ultrasound. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aaf6086
Idbaih A, Canney M, Belin L et al (2019) Safety and feasibility of repeated and transient blood–brain barrier disruption by pulsed ultrasound in patients with recurrent glioblastoma. Clin Cancer Res 25:3793–3801. https://doi.org/10.1158/1078-0432.CCR-18-3643
Asquier N, Bouchoux G, Canney M et al (2020) Blood–brain barrier disruption in humans using an implantable ultrasound device: quantification with MR images and correlation with local acoustic pressure. J Neurosurg 132:875–883. https://doi.org/10.3171/2018.9.JNS182001
Park SH, Kim MJ, Jung HH et al (2020) One-year outcome of multiple blood–brain barrier disruptions with temozolomide for the treatment of glioblastoma. Front Oncol. https://doi.org/10.3389/fonc.2020.01663
Park SH, Kim MJ, Jung HH et al (2021) Safety and feasibility of multiple blood–brain barrier disruptions for the treatment of glioblastoma in patients undergoing standard adjuvant chemotherapy. J Neurosurg 134:475–483. https://doi.org/10.3171/2019.10.JNS192206
Mainprize T, Lipsman N, Huang Y et al (2019) Blood–brain barrier opening in primary brain tumors with non-invasive MR-guided focused ultrasound: a clinical safety and feasibility study. Sci Rep 9:321. https://doi.org/10.1038/s41598-018-36340-0
Anastasiadis P, Gandhi D, Guo Y et al (2021) Localized blood–brain barrier opening in infiltrating gliomas with MRI-guided acoustic emissions-controlled focused ultrasound. Proc Natl Acad Sci. https://doi.org/10.1073/pnas.2103280118/-/DCSupplemental
Chen K-T, Lin Y-J, Chai W-Y et al (2020) Neuronavigation-guided focused ultrasound (NaviFUS) for transcranial blood–brain barrier opening in recurrent glioblastoma patients: clinical trial protocol. Ann Transl Med 8:673. https://doi.org/10.21037/atm-20-344
Chen K-T, Chai W-Y, Lin Y-J et al (2021) Neuronavigation-guided focused ultrasound for transcranial blood–brain barrier opening and immunostimulation in brain tumors
Wei K-C, Chu P-C, Wang H-YJ et al (2013) Focused ultrasound-induced blood–brain barrier opening to enhance temozolomide delivery for glioblastoma treatment: a preclinical study. PLoS ONE 8:e58995. https://doi.org/10.1371/journal.pone.0058995
Liu H-L, Hua M-Y, Chen P-Y et al (2010) Blood–brain barrier disruption with focused ultrasound enhances delivery of chemotherapeutic drugs for glioblastoma treatment. Radiology 255:415–425. https://doi.org/10.1148/radiol.10090699
Aryal M, Vykhodtseva N, Zhang Y-Z et al (2013) Multiple treatments with liposomal doxorubicin and ultrasound-induced disruption of blood-tumor and blood–brain barriers improve outcomes in a rat glioma model. J Control Release 169:103–111. https://doi.org/10.1016/j.jconrel.2013.04.007
Ortiz R, Perazzoli G, Cabeza L et al (2020) Temozolomide: an updated overview of resistance mechanisms, nanotechnology advances and clinical applications. Curr Neuropharmacol 19:513–537. https://doi.org/10.2174/1570159x18666200626204005
Adkins CE, Mittapalli RK, Manda VK et al (2013) P-glycoprotein mediated efflux limits substrate and drug uptake in a preclinical brain metastases of breast cancer model. Front Pharmacol. https://doi.org/10.3389/fphar.2013.00136
Cho H, Lee H-Y, Han M et al (2016) Localized down-regulation of P-glycoprotein by focused ultrasound and microbubbles induced blood–brain barrier disruption in rat brain. Sci Rep 6:31201. https://doi.org/10.1038/srep31201
Zhang DY, Dmello C, Chen L et al (2020) Ultrasound-mediated delivery of paclitaxel for glioma: a comparative study of distribution, toxicity, and efficacy of albumin-bound versus Cremophor formulations. Clin Cancer Res 26:477–486. https://doi.org/10.1158/1078-0432.CCR-19-2182
Chang SM, Kuhn JG, Robins HI et al (2001) A Phase II study of paclitaxel in patients with recurrent malignant glioma using different doses depending upon the concomitant use of anticonvulsants. Cancer 91:417–422. https://doi.org/10.1002/1097-0142(20010115)91:2%3c417::AID-CNCR1016%3e3.0.CO;2-9
O’reilly MA, Hynynen K (2012) Blood–brain barrier: real-time feedback-controlled focused ultrasound disruption by using an acoustic emissions-based controller. Radiology. https://doi.org/10.1148/radiol.11111417
Sheikov N, McDannold N, Sharma S, Hynynen K (2008) Effect of focused ultrasound applied with an ultrasound contrast agent on the tight junctional integrity of the brain microvascular endothelium. Ultrasound Med Biol 34:1093–1104. https://doi.org/10.1016/j.ultrasmedbio.2007.12.015
Arvanitis CD, Crake C, McDannold N, Clement GT (2017) Passive acoustic mapping with the angular spectrum method. IEEE Trans Med Imaging 36:983–993. https://doi.org/10.1109/TMI.2016.2643565
Jones RM, O’Reilly MA, Hynynen K (2013) Transcranial passive acoustic mapping with hemispherical sparse arrays using CT-based skull-specific aberration corrections: a simulation study. Phys Med Biol 58:4981–5005. https://doi.org/10.1088/0031-9155/58/14/4981
Salgaonkar VA, Datta S, Holland CK, Mast TD (2009) Passive cavitation imaging with ultrasound arrays. J Acoust Soc Am 126:3071–3083. https://doi.org/10.1121/1.3238260
Bae S, Liu K, Pouliopoulos AN et al (2023) Real-time passive acoustic mapping with enhanced spatial resolution in neuronavigation-guided focused ultrasound for blood–brain barrier opening. IEEE Trans Biomed Eng 70:2874–2885. https://doi.org/10.1109/TBME.2023.3266952
Wu S-K, Chu P-C, Chai W-Y et al (2017) Characterization of different microbubbles in assisting focused ultrasound-induced blood–brain barrier opening. Sci Rep 7:46689. https://doi.org/10.1038/srep46689
Chai W-Y, Chu P-C, Tsai M-Y et al (2014) Magnetic-resonance imaging for kinetic analysis of permeability changes during focused ultrasound-induced blood–brain barrier opening and brain drug delivery. J Control Release. https://doi.org/10.1016/j.jconrel.2014.06.023
Syed HR, Kilburn L, Fonseca A et al (2023) First-in-human sonodynamic therapy with ALA for pediatric diffuse intrinsic pontine glioma: a phase 1/2 study using low-intensity focused ultrasound: technical communication. J Neurooncol 162:449–451
Rezai AR, Ranjan M, Haut MW et al (2023) Focused ultrasound–mediated blood–brain barrier opening in Alzheimer’s disease: long-term safety, imaging, and cognitive outcomes. J Neurosurg 139:275–283. https://doi.org/10.3171/2022.9.JNS221565
Abrahao A, Meng Y, Llinas M et al (2019) First-in-human trial of blood–brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat Commun. https://doi.org/10.1038/S41467-019-12426-9
Batchelor TT, Mulholland P, Neyns B et al (2013) Phase III randomized trial comparing the efficacy of cediranib as monotherapy, and in combination with lomustine, versus lomustine alone in patients with recurrent glioblastoma. J Clin Oncol 31:3212–3218. https://doi.org/10.1200/JCO.2012.47.2464
Hutterer M, Nowosielski M, Haybaeck J et al (2014) A single-arm phase II Austrian/German multicenter trial on continuous daily sunitinib in primary glioblastoma at first recurrence (SURGE 01–07). Neuro Oncol 16:92–102. https://doi.org/10.1093/neuonc/not161
Kalpathy-Cramer J, Chandra V, Da X et al (2017) Phase II study of tivozanib, an oral VEGFR inhibitor, in patients with recurrent glioblastoma. J Neurooncol 131:603–610. https://doi.org/10.1007/s11060-016-2332-5
Van Den Bent M, Eoli M, Sepulveda JM et al (2020) INTELLANCE 2/EORTC 1410 randomized phase II study of Depatux-M alone and with temozolomide vs temozolomide or lomustine in recurrent EGFR amplified glioblastoma. Neuro Oncol 22:684–693. https://doi.org/10.1093/neuonc/noz222
Kong C, Chang WS (2023) Preclinical research on focused ultrasound-mediated blood–brain barrier opening for neurological disorders: a review. Neurol Int 15:285–300. https://doi.org/10.3390/neurolint15010018
Acknowledgements
The authors would like to acknowledge funding support from Children’s Cancer and Leukaemia Group (CCLG)/Little Princess Trust (CCLGA 2022 25), Action Medical Research/LifeArc (GN3017), the Focused Ultrasound Foundation (FUS1050R1), and Abbie’s Army/Children’s Brain Tumor Drug Delivery Consortium (KCL/G12/22).
Funding
The authors have not disclosed any funding.
Author information
Authors and Affiliations
Contributions
H.Z. and C.A. contributed equally to this work and share first authorship. H.Z. and C.A. wrote the main manuscript text and prepared figures and tables. A.N.P. supervised the project. All authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, H., Allwin, C., Bassous, M.G. et al. Focused ultrasound-mediated enhancement of blood–brain barrier permeability for brain tumor treatment: a systematic review of clinical trials. J Neurooncol (2024). https://doi.org/10.1007/s11060-024-04795-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11060-024-04795-z