Abstract
Glioblastoma (GB) is one of the most aggressive and difficult-to-treat brain tumors, with a poor prognosis and limited treatment options. In recent years, sonodynamic therapy (SDT) and magnetic resonance focused ultrasound (MRgFUS) have emerged as promising approaches for the treatment of GB. SDT uses ultrasound waves in combination with a sonosensitizer to selectively damage cancer cells, while MRgFUS delivers high-intensity ultrasound waves to precisely target tumor tissue and disrupt the blood–brain barrier to enhance drug delivery. In this review, we explore the potential of SDT as a novel therapeutic strategy for GB. We discuss the principles of SDT, its mechanisms of action, and the preclinical and clinical studies that have investigated its use in Gliomas. We also highlight the challenges, the limitations, and the future perspectives of SDT. Overall, SDT and MRgFUS hold promise as novel and potentially complementary treatment modalities for GB. Further research is needed to optimize their parameters and determine their safety and efficacy in humans, but their potential for selective and targeted tumor destruction makes them an exciting area of investigation in the field of brain cancer therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gliomas represent about 25% of all primary brain tumors, encompassing malignant and not malignant subtypes [1]. IDH-wildtype glioblastoma (2022 WHO grade 4) exhibited the highest age-adjusted incidence rates and is considered the most aggressive variant, characterized by an extremely aggressive biological behavior resulting in a poor outcome [2]. Despite the enormous progress in biotechnology and medicine field, life expectancy of glioblastoma (GB) patients has improved only slightly over the last 30 years.
As a matter of fact, if untreated, GB’s median survival means is 3 months [3]. Interestingly, the standard management of GB has not changed since Roger Stupp published his work [4].
Recently, supramarginal resection or, where possible, excision of the hyperintense area in FLAIR sequences in MRI (so-called Flair-ectomy) has been shown to be associated with improvement in both overall survival (OS) and progression free survivor (PFS), although not always executable, especially in lesions involving eloquent brain areas [5, 6].
Other therapeutic strategies tested included anti-angiogenic therapy and immunotherapy, though they did not show significant improvement in OS [7]. Some authors also advocated the importance of palliative care to increase the quality of life in patients affected from this tremendous tumor [8, 9].
In the era of genomic and molecular genetics, in which it is possible to investigate all the potential metabolic landscape of the disease, new treatment strategy is going to rely more on biochemical and immunological treatments [10,11,12].
Various new treatments have been proposed over the years, regarding use of CAR T cells [13], molecular agents enhancing the effect of radiotherapy (RT) [14], up to the application of high and low intensity focus ultrasound [15].
In this context, sonodynamic therapy (SDT) seems to become a promising treatment, offering the possibility of non-invasively eradicate solid tumor in a site-directed manner, employing compounds that become cytotoxic after being exposed to low intensity ultrasound [16]. The importance of this kind of treatment could gain an added value considering the possibility of deep lesion-targeting thanks to the significant depth that low-intensity ultrasound penetrates tissue, not damaging surround brain parenchyma and the chance of aiming directly to cancer stem cells (found to be vital cells for proliferation, differentiation, and treatment resistance of the GB) [17,18,19,20]
Remarkably, the possibility of impairing the brain blood barrier (BBB) is another weapon in neurosurgical armamentarium, making easier the access of chemical agents [20, 21].
Our review aims to provide a current view of the use of focused ultrasound, and particularly SDT, in the management of GB, starting with their mechanism of action in vitro and in animal models and ending with current or future clinical trials, exploring the limitations and potential of such treatment.
Materials & Methods
Search of the literature
Preferred Reporting Items for Systematic reviews and Meta-analyses guidelines (PRISMA) were followed to conduct and report this systematic literature review [22] (Fig. 1).
We performed a broad systematic literature search in different online scientific libraries (Pubmed/MEDLINE, Cochrane Library and ClinicalTrial.Gov) for all studies investigating the efficacy and feasibility of SDT in GB treatment. The protocol of this review has been prospectively registered in Open Science Framework and it is publicly available online at https://doi.org/10.17605/OSF.IO/FW4QS.
We searched for studies published up to the 15th of September 2022 without backward limits, using the following MeSH terms “Glioblastoma” AND “Focused Ultrasound”, “Glioblastoma” AND “FUS”, “Glioblastoma” AND “MRgFUS”, “Glioblastoma” AND “Sonodynamic therapy“, “Glioblastoma” AND “High intensity Focused Ultrasound”, “Glioblastoma” AND “HIFU”, “Glioblastoma” AND “Sonodynamic” AND “therapy”, “Gliomas” AND “Sonodynamic therapy“, “Glioblastoma” AND “focused ultrasound” AND “sonosensitizer”, “Glioma” AND “focused ultrasound” AND “sonosensitizer”.
To avoid the potential omission of relevant studies we also manually screened reference lists of articles included and previous systematic reviews and meta-analyses regarding the topic. Duplicate articles were eliminated using Microsoft Excel 16.37 (Redmond, WA, USA).
Study selection
The studies included in our paper were both studies in vitro and in vivo using animal models, and ongoing clinical trials. The proof-of-concept of our research was trying to understand the effect of SDT on Glioma/GB cell line and then the feasibility, efficacy, and safety of its application firstly in animal model and then in the clinical practice, evaluating the ongoing clinical trials.
The research strategy initially relied on title and abstract analysis. The article’s full text was retrieved for further investigation if the title and abstract met the inclusion criteria. The data collection process was conducted without using any automated tools. The research was conducted by two different authors separately (U.E.B and K.G) and eventually refined by a third author (L.B). No ethical approval was required for this study.
Eligibility criteria
The articles were selected according to the following inclusion criteria:
-
Full article in English.
-
Studies in a preclinical phase (‘in vitro’ and ‘in vivo’ study).
-
Case report, case series, retrospective study, prospective study and clinical trials.
-
Patients age ≥ 18.
-
Patients affected by glioblastoma treated with SDT.
Exclusion criteria:
-
Articles not in English.
-
Editorials, books, systematic reviews, and meta-analysis.
-
Patients age < 18.
-
Patients treated with focused ultrasound used to perform a disruption in brain-blood-barrier (BBB).
-
Studies evaluating focused ultrasound therapy but not focusing on SDT.
Data extraction
According to the criteria above, all articles were identified by two reviewers (K.G. and U.E.B.). In case of a discrepancy, a third author (L.B.) arbitrated until a consensus among the authors was reached.
The extracted data included the following: publication’s year, author, study design, patients’ number, patients’ mean age and gender, type of cells or animals, aim of the study and results of the study.
Results
Data selection
Our initial research carried out through Pubmed identified a total of 373 articles. We excluded 128 duplicated articles, then we performed a further screening based upon title and abstract reading, eliminating 157 articles.
Finally, after a full text reading and a detailed examination, 65 articles were excluded, because either they were focusing only on the effect of focused ultrasound on BBB disruption (39 articles) or they were reviewing previous scientific works (16 articles) or lastly because they were not inherent with the purpose of this review, including finally 23 studies in our systematic review, according to PRISMA flow diagram inclusion criteria.
The characteristics of included articles are the following ones: publication’s year, author, study design, type of cells and animal model (respectively for the ‘in vitro’ and for the ‘in vivo’ studies), aim of the study and results of the study.
The articles were eventually divided in three tables, including studies ‘in vitro’ and studies ‘in vivo’ on animal models (Tables 1, 2).
Using “Sonodynamic Therapy“ AND “Glioblastoma” and “Focused Ultrasound” AND “Glioblastoma” as MeSH terms, another research on ClinicalTrial.Gov was performed, identifying a total of 11 trials. After exclusion criteria were applied, only four trials were included in the review.
The characteristics of included trials are the following ones: title of the trial, identifier, status, interventions characteristics, aim of the study and locations of the trial (Table 3).
Study characteristics
In vitro' and in vivo' studies
Tumor cell lines used in the listed studies were both murines and humans: rat C6 glioma cells were the most used ones, followed by human U87 GB cells and other cell lines such as human glioma cells U373, U105MG and U251MG.
‘In vivo’ studies used as animal models mainly murines, both mice and rats; only one study employed a porcine model.
Both ‘in vitro’ and ‘in vivo’ studies used different types of sonosensitizers, such as 5-Aminolevulinic acid hydrochloride (5-ALA), sinoporphyrin sodium (DVDMS), hematoporphyrin monomethyl ether (HMME), temozolomide (TMZ), photofrin, fluorescein (FL) and disodium tetraiodo tetrachloro fluorescein (Rose Bengal), as a way of increasing the tumoral cells’ vulnerability to focused ultrasounds’ exposition.
‘In vitro’ studies focused their attention on the effect that ultrasound, with or without the use of a sonosensitizer, provoked on tumoral cell lines, in term of apoptotic rate and intracellular level of reactive oxygen species (ROS) post-exposition; moreover, the minimal intensity of ultrasound in order to produce an apoptotic effect on tumoral cell was also investigated [23]. Some studies also tried to quantify the anti-tumoral effect of ultrasound with or without the use of a sonosensitizer [26].
Moreover, Gonzales et. al. proved the increased efficacy of STD in combination with bleomycin, in its inhibition of tumoral growth.
‘In vivo’ studies used an animal model to verify the feasibility of this technique: more than investigating just the anti-tumoral effect of STD, these studies prove the safety of SDT towards healthy brain tissue [33, 45].
Other information obtained from ‘in vivo’ studies regard the efficacy of focused ultrasound used in combination of a sonosensitizer in inducing tumor growth inhibition and the underlying physiopathological mechanism, described thanks to post-autoptic histology. STD therapy was able to induce an increased apoptotic rate, through an increased ROS production, reduced production anti-apoptotic/pro-angiogenic factors and microvessel destruction [37].
Many of the ‘in vivo’ studies were coupled to in vitro experiments where the same method was tested, reporting analogies and differences in both results; some studies, instead, were performed directly ‘in vivo’, on animal models. The studies reviewed used similar sonication parameters regarding intensity of sonication performed (range from 0.2 to 25 W/cm2). The frequency used in the studies ranged from 0.5 to 3 MHz. The maximum value of the duration was 20 min. All the studies included, especially ‘in vivo’ studies, have demonstrated that SDT are effective in reducing tumor volume, because of its high selectivity, low toxicity, and deep penetration, focusing on both the ability to reduce tumor growth and placing emphasis on the survival of tumor cells after the treatment (Tables 1, 2).
Clinical trials
We identified four clinical trials about SDT in GB treatment. Between them, it is listed a non-randomized, single-arm study whose purpose is to evaluate the safety and feasibility of SDT with 5-aminolevulinic acid in patients with newly diagnosed cerebral GBs using the ExAblate Model 4000 Type-2 Neuro System.
Another clinical trial, non-randomized, tried to assess the safety, dose limiting toxicities, and preliminary efficacy of SDT using SONALA-001 and Exablate Type-2 device in subjects with recurrent or progressive GB.
Additionally, a phase 1 multi-center trial started to understand the safety and tolerability of 5-aminolevulinic acid (5-ALA) combined with CV01 delivery of ultrasound for SDT in patients with recurrent high-grade glioma.
Finally, we report a phase 0 single-center open label study whose intention is to appraise the ascending energy doses of SDT utilizing the MRgFUS combined with intravenous 5-ALA and its efficacy in patients with recurrent HGG.
Studies characteristics and aim were summarized in Table 3.
Discussion
Conventional therapeutic options in the treatment of solid brain tumors, and GBs in particular, are based on the assumption that these cancerous lesions have relatively homogeneous spatiotemporal characteristics. However, recent advances in the molecular, genetic, and epigenetic fields have shown how this does not reflect the facts at all, underscoring the inherent limitations of radiation and chemotherapy [46,47,48]. Moreover, the notion that GBs do not represent a focal pathological entity, but rather a pathology spread throughout the entire brain, makes clear the inherent limitations of surgery, although it still represents the therapeutic mainstay toward these tumors [49, 50].
Hence the need to develop new therapeutic strategies capable of eradicating the underlying pathology, possibly in the least invasive way, and increasing OS and PFS while safeguarding patients' quality of life and neurological status [51, 52].
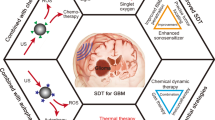
In this context, the use of ultrasound for therapeutic purposes (the so-called Theranostics) appears to offer interesting potential and promising results and uses [53].
As a matter of fact, focused ultrasound can be employed either to destroy cancerous cells by heating or as an adjuvant therapy, in combination with chemotherapy or radiation therapy. The main points of values of FUS are the non-invasiveness, incision-free, controllability via real-time MR guidance and the capacity to activate the immune system [54]. The first non-invasive thermal ablation of a brain tumor in human was realized by Coluccia et al. [55] in their ongoing clinical phase I study in 2014, when they firstly employed Magnetic resonance-guided focused ultrasound surgery (MRgFUS) for safe thermal ablation of a centrally located recurrent GB. This is possible thanks to recent advances in magnetic resonance imaging, which allow safe and precise thermal ablation of neoplastic tissue. Moreover, the opportunity to create an MRI-derived temperature mapping of the targeted tissue allow a non-invasive monitoring of the ablating procedure. In more recent years, knowledge about the different mechanisms of action of ultrasound at various intensities and frequencies, used alone or in combination with other substances, has been expanded, exploring new potentials, and developing new therapeutic strategies, including precisely SDT [56,57,58,59,60,61].
Sonodynamic therapy
SDT has been developed as a promising tool in brain tumor treatment. SDT takes its cue from photodynamic therapy (PDT), in which a light-activated photosensitizer can cause the generation of ROS, which in turn would mediate a cytotoxic effect on neoplastic cells. However, the main limitation of PDT is the range of action, which is limited to superficial lesions due to the poor penetration of laser light into brain tissue [42, 62]. This obstacle is overcome using low-intensity ultrasound, which has a greater penetrative capacity [63].
As just mentioned, SDT involves the application of focused ultrasound with a substance that sensitizes cells to the destroying effects of sound, called sonosensitizer. It includes both ultrasonication, via non-invasive low-intensity ultrasound penetrating soft tissues and focus on a specific site, and sonosensitizers, which embrace non-toxic chemical agents such as 5-ALA, ATX-70, Hypocrellin, Rose Bengal and many others [64,65,66]; some of these compounds are commonly used in glioma surgery to intraoperatively visualize the tumor and can be employed to induce cytotoxic effects to neoplastic cells when subjected to a specific acoustic field [31, 39, 40]. The advantage of this technique is to minimize adverse events and maximizing on-target responses. Furthermore, the use of chemical agents that are non-toxic in the absence of a specific stimulus distinguishes the definition of SDT from the broader meaning of FUS employed to enhance the effects of an already toxic compound [67, 68].
Sheehan et colleagues [69] employed the SDT on two cellular lines, rat C6 and human U87 GB cells, and found that two innocuous agents, which are FUS and 5-ALA, can lead to cell death by the transformation of 5-ALA to PPIX in malignant glioma cells, where it generates reactive oxygen species responsible of cellular apoptosis. ‘In vivo’ studies have proved that focused ultrasound in combination with the systemic administration of 5-ALA is effective in treating intracranial gliomas in rats, not demonstrated by the complete tumor resection but by the reduction in tumor size from the initial tumor volume [34, 35, 37, 39]. In this regard, Nonaka et al. [33] pinpointed the optimal focused ultrasound acoustic energy and duration for the ablation of brain tumor in rats, without damaging normal brain tissue; in their experience, this selective anti-tumor effect was produced by weaker focused ultrasound intensity at 25 W/cm2 at 1 MHz for 5 min.
In 2019, Abdolhosseinzadeh et al. [70] investigated the effects of focused acoustic waves in a focal area through some accurate simulations. Results obtained in 2D, and 3D models showed that ultrasound waves could be used in the form of pulse waves with different time periods to provoke a focused thermal lesion on neoplastic tissue [71, 72]. Nevertheless, ‘in vivo’ it is necessary to overcome the blood brain barrier (BBB), which represents a real obstacle to sonosensitizers. To this aim, low-intensity focused ultrasound can be combined to microbubbles, which proved to increase the permeability of the BBB, allowing the treatment of intracranial GB in mice. These results can suggest the use of SDT with sonosensitizers in human GB [73,74,75].
Sonosensitizers
As previously mentioned, the sonosensitizers used in SDT are harmless molecules that when subjected to an acoustic field mediate a cytotoxic effect. Many of these molecules are the same as those used in photodynamic therapy and are agents based on porphyrin or related molecules (protoporphyrin IX, hematoporphyrin, etc.). In fact, there is evidence that such molecules, when exposed to the action of ultrasound, result in the production of reactive oxygen species (ROS). In their ‘in vitro’ study, Shen et al. [32] employed as a sonosensitizer the sinoporphyrin sodium, purified from photofrin II, which showed great antitumor effect on human GB cell lines; particularly, this sonosensitizer can easily enter in cancer cells and accumulate into the mitochondria, where it gives raise to cytotoxity through the production of ROS.
However, although these agents are preferentially picked up by the tumor, they exhibit marked hydrophobicity, and their distribution appears to be ubiquitous [76]. Despite this apparently drawback, it has been postulated by Raspagliesi and colleagues that three contemporary events must occur to determine a cytotoxic effect: the administration of ultrasound, the administration of a sonosensitizer, and the presence of a lesion where the latter can reach a significant concentration. This concept led to the non-invasive effect on SDT on normal brain tissue, since, even if the sonosensitizer has been collected in healthy tissue, it would be inconsequential [45].
Thus, it seems clear that the choice of sonosensitizer is also crucial. Ideally, the perfect sonosensitizer should exhibit high affinity for tumor cells and slow clearance from the neoplasm, while sparing healthy brain parenchyma [77,78,79].
Mechanism of action of sonodynamic therapy
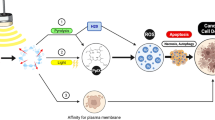
The effects of thermal ablation on tumoral cells are still not completely clear. Is has been demonstrated that hyperthermia (HT) can enhance the 5-ALA-SDT induced cell apoptosis partly by activating caspases and by modulating Bcl-2 family members. Moreover, HT is responsible of increasing ROS production and reducing metalloproteases (MMPs) induced by 5-ALA-SDT in human glioma cells [38, 80] (Figs. 2, 3).
Representation of 3 mechanisms of sonodynamic therapy: A 5 ALA SDT could reduce level of Bcl2, thus activating apoptosis via caspase 9 pathway. B 5 ALA SDT increase levels of ROS, thus inducing cell death. C heating itself could increase level of Heat shock protein 70, thus inducing immune response through structural changing in cell membrane
HT can also regulate some molecular aspects of the immune response, such as Fas gene and its ligand FasL, and act as an immunomodulator in cancer therapy [81, 82]. In more details, it seems that the increasing in local temperature may act as a natural trigger or danger signal to the immune system. Hyperthermia can therefore enhance the expression of FAS-ligand mRNA, which has a role in functional maturation of dendritic cells together with secretion of proinflammatory cytokines, which in turn activate T lymphocytes and induce a polarization toward a Th1 phenotype. Moreover, high temperature may promote the action of a particular set of protein, the HSPs, which may act in protecting cells from dangerous stress by regulating cell homeostasis [83] and may also affect the stability of cellular membranes by inducing structural changing that intervene in signaling events and cell migration in immune response [84, 85].
The link between ultrasound exposure, presence of sonosensitizer and generation of ROS appear clear, and there is a consensus regarding their involvement in mediating the cytotoxic effect on cancer cells [86,87,88]. Nonetheless, other mechanisms have been proposed to elucidate the SDT-mediated cytotoxic effect. These include sonoluminescence, namely the emission of light from cavitation bubbles, which would appear to play a role both in the activation of certain sonosensitizers and in mediating antitumor effects [89, 90], and sonomechanical mechanisms that would mediate damage by inducing changes at the level of cell membranes, such as a reduction in membrane fluidity and an increase in lipid peroxidation [91, 92]. Noteworthy are the various cytotoxic actions and implicated mechanisms that characterize the different sonosensitizers, although further studies on this are needed [93, 94].
Interestingly, SDT can also be used in combination with other agents commonly used to treat GB to enhance their action, such as the temozolomide (TMZ). Resistance of high-grade glioma cells to TMZ is related to high level expression of NHE-1 protein, which enhance cells invasion to normal brain tissue. In their article, Chen et al. [27] demonstrated that SDT can suppress NHE-1 expression, thus allowing the cytotoxic effect of TMZ in vitro.
Another target to take advantage of to enhance the anti-tumor efficacy of TMZ is the p-glycoprotein, referred to as multidrug resistance receptor (MDR1), a transmembrane protein which act as an efflux pump and confer multidrug resistance in brain tumor. Consequently, high expression of MDR1 is present in resistant GB and the downregulation of MDR1 via Akt/NF-kB pathway can improve the antitumor effect of temozolomide in GB cells. Shono et al. [31] demonstrated that the elevation of cellular PpIX using celecoxib is related to a down regulation of Akt/NF-kB/MDR1 pathway, thus enhancing the anti-tumor efficacy of SDT. Some authors advocated that the SDT mediated by hematoporphyrin monomethyl ether (HMME) can induce apoptosis on C6 glioma cells in vitro and suggest that the mitochondrial signal pathway may play a pivotal role, because of the observed production of ROS, loss of MMP and Bcl-2 and protein expression in caspace-9, caspase-3 and Bax [25, 29].
Ultrasound parameters affecting SDT results
Although the exact mechanism of action of SDT is not yet fully understood, it is assumed that the biological effects of this technique are strongly correlated with the phenomenon of acoustic cavitation (stable vs. inertial cavitation) derived from the interaction between ultrasound and the propagation medium, ultimately resulting in apoptosis of the affected cells. In addition to the mechanical effect of ultrasound, the action of SDT is also based on the sonochemical effect related to the formation of various species of free radicals and the different decomposition kinetics of sonosensitizers [63, 95, 96]. These various mechanisms of action in turn are closely related to the ultrasound parameters used and to other factors associated to the experimental setting (see Table 4).
For instance, it has been demonstrated that most sonosensitizers respond to US frequency ranging from 0.2 to 3 MHz [42] and that a decrease in frequency is correlated to an increase in ultrasound toxicity [25]. However recent works have pointed out apoptotic cell ratio was primarily affected by sonosensitizer concentration and then by other variables such as US frequency, irradiation time and intensity [97, 98]. US intensity usually ranges from 0.5 to 10W/cm2 and can be applied in a continuous or pulsatile mode [96]. Regarding this parameter, many studies have noted an intensity-dependent reduction in cell viability of various cancer types [99, 100]. Nejad et al. [101] have shown in a model of human oral squamous cell line HSC-2 how irradiating cells with 3.5 MHz US at 20, 32, 55, and 73 W/cm2 was associated to a cell survival rate of 97, 81, 62 and 40%, respectively.
Irradiation time and duty cycle also influenced SDT results, with greater citotoxity at greater irradiation time and duty cycle [102]. Besides US parameters, many other factors may influence SDT response ‘in vitro’ studies. Irradiation uniformity and intensity distribution, for example, are related to the distance between cells and US apparatus, as well as the characteristics of the coupling media and culture medium also seem to profoundly impact the outcomes of SDT. Other two crucial factors that affect the results of SDT are the type of irradiated sample and the sonosensitizer concentration, the latter closely related to the apoptotic effect, as shown by Zhang et al. [103,104,105].
Ongoing clinical trials
Evidence regarding the potential application of SDT in high-grade gliomas are mostly taken from pre-clinical ‘in vitro’ and ‘in vivo’ studies. Currently, there are four ongoing trials concerning the role of SDT in high grade gliomas registered on “Clinicaltrial.gov”, of which three are recruiting.
The aim of the first trial is to evaluate the safety and tolerability of 5-ALA combined with CV01 delivery of ultrasound in patients affected by recurrent high-grade gliomas. (ClinicalTrials.gov Identifier: NCT05362409). This ongoing phase 1 trial is recruiting 33 patients, to which 5-ALA will be administered as sonosensitizer prior to CVo1-delivered ultrasound, which will deliver non-ablative, low-intensity ultrasound; 5-ALA will be then re-administered every 4 weeks prior to CV01. The primary outcome is to evaluate the incidence of adverse events and to determine the Maximum Tolerable Duration in the first 12 months. Secondary outcome is represented by the assessment of Overall Response Rate, Duration of Response, OS and PFS in the first 12 months.
The second recruiting trial (ClinicalTrials.gov Identifier: NCT05370508) aims to evaluate the safety and preliminary efficacy of SDT by using SONALA-001 as sonosensitizer and Exablate 4000 Type-2 MR-guided focused ultrasound as device in people affected by recurrent or progressive GB. The primary outcomes are represented by the evaluation of the safety of SDT in the first 12 months, the maximum tolerable duration in the first 29 days, the determination of recommended phase 2 schedule and the assessment of progression free survival in the first 6 months.
The third prospective, non-randomized, single-arm, not yet recruiting study (ClinicalTrials.gov Identifier: NCT04845919) aims to evaluate the safety and feasibility of SDT by using 5-ALA and Exablate 4000 Type-2 MRgFUS in patients newly diagnosed with GB. Patients screened will undergo SDT treatment, will perform a strict neuro-radiological follow-up after the procedure (minimum 2 MRI) and will undergo tumor resection 14–21 days after SDT. The primary outcome is represented by the early identification of hemorrhage, oedema, or other damages in the first 10 days; secondary outcomes are represented by the evaluation of the rate of neurological deficits and the radiological response to treatment in the first 10 days after the procedure.
The last nRCT (identifier: NCT 04559685) is a Phase 0 single center, first in human, open-label study of ascending energy doses of SDT utilizing the MRgFUS combined with intravenous ALA to assess safety and efficacy in up to 30 participants with recurrent HGG. The primary outcomes are to assess the biological changes associated with the SDT, analyzing the percentage of Cleaved Caspase-3, MIB-1 level and GammaH2Ax of the surgical specimen. Secondary outcomes include the evaluation of radiographic evidence of tumor physiological imaging changes and the assessment of performance, safety and tolerability of the MRgFUS and SDT.
Conclusions: challenges, limits, and future directions
This review explored the current literature regarding the role of SDT in glioma treatment, and particularly in GB, considering the evidence from ‘in vitro’ and ‘in vivo’ studies, and the ongoing clinical trials on its clinical human application. We also focused on the possible mechanisms of action underlying SDT and the role of different sonosensitizers. The study of the latter seems to enshrine the marriage of SDT and nanomedicine, paving the way for future research and new possibilities.
Based on the studies that have been discussed on this paper and the current ongoing trials, SDT could be a valuable option in patients with GB, due to the opportunity to induce toxicity only in a precise localization while minimizing harm in normal areas. Indeed, thanks to the development of increasingly sophisticated and accurate software is possible to target tumor volume precisely. In addition, nanotechnology-based drug delivery systems have been developed to enhance the selective accumulation of the sonosensitizer in tumor cells. Actually, SDTseems to be more effective in treating GB than low-grade glioma (LGG). This is because GB cells are more susceptible to the effects of SDT due to their higher rate of metabolism and greater degree of angiogenesis compared to LGG cells [38, 106, 107]. However, more research is needed to confirm these findings and determine the optimal parameters for SDT in the treatment of different types of brain tumors. Furthermore, the effectiveness of SDT may also depend on other factors such as tumor size, location, genetic characteristics, and vascular pattern. For instance, brain tumors located near the skull base may be more amenable to SDT. The size of the tumor can also affect the effectiveness of the procedure. Larger tumors may be more difficult to treat with SDT, as the ultrasound waves may not be able to penetrate deep enough into the tumor to effectively kill the cancer cells.
Limitation on the clinical application rely on the fact that SDT represents a novel technique that needs to be further investigated. First, the role of sonosensitizers should be deepened: many sensitizers are employed in both PDT and SDT and residuals can accumulate in areas other than tumors, thus leading to hypersensitivity to light. Strategies to overcome this limitation are therefore needed, such as the opportunity to employ microbubbles to carry sonosensitizer or to employ new sensitizers specific for the SDT. Moreover, attention should be payed to the phenomenon of cavitation, which enables the sonochemical reactions to occur. Initiation of cavitation can be difficult, because of the high pressure required. Authors suggested some strategies to facilitate the cavitation, such as the application of standing waves rather than progressive or the dual frequency sonication [96].
Other major concerns are that US procedures require long treatment sessions, therefore confining its application to small volumes, and the lack of in-vivo studies; in this regard efforts have been made and several ongoing clinical and pilot trials aim to better define the real clinical employment of SDT in patients affected by GB [108].
Challenges are also represented by the correct application into the neurooncological field of devices currently employed for other neurological disorders; an example is given by the essential tremor which benefit from the MRgFUS performed with the ExAblate Neuro 4000. It is therefore necessary to adapt and modify some characteristic such as the frequencies employed, which are lower in the setting of a tumor compared to the treatment of essential tremor (220 KHz vs 650 KHz) [109].At present, SDT is not indicated as a first-line treatment for GB or any other type of brain cancer.
However, several scenarios are still open: SDT may have potential as an adjunctive treatment to enhance the effectiveness of standard therapies, such as chemotherapy via chemosensitization effect, or as a salvage option for patients who have failed other treatments, alone or in combination to other techniques to enhance its effect (e.g. hyperthermotherapy). In some cases, it may also be considered as a primary treatment strategy for patients who are not suitable candidates for surgery or who have recurrent tumors that are difficult to treat with other modalities.
Further studies are certainly needed to better define the role of sonodynamic therapy in these patients and, particularly, the eligibility criteria for this treatment, such as the stage of disease (i.e., primary, or recurrent GB) and the opportunity to employ SDT as a first line treatment or as a palliative strategy, as well as patient condition, such as KPS or current comorbidities. Finally, the opportunity to use this technique in brain tumors other than gliomas should be deepened: over the last years new indications have been considered as potential targets of the ultrasound therapy, such as brain metastasis (from breast cancer or melanoma), neuroblastoma, neurofibromatosis, astrocytomas and pontine gliomas, and both preclinical and clinical trials are ongoing [108].
Data availability statement
All data generated or analyzed during this study are included in this published article.
References
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro Oncol 23:1–105. https://doi.org/10.1093/neuonc/noab200
Iorgulescu JB et al (2022) Molecular biomarker-defined brain tumors: epidemiology, validity, and completeness in the United States. Neuro Oncol. https://doi.org/10.1093/neuonc/noac113
Malmström A et al (2012) Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, Phase 3 Trial. Lancet Oncol 13:916–926. https://doi.org/10.1016/S1470-2045(12)70265-6
Stupp R et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Certo F et al (2020) Supramarginal resection of glioblastoma: 5-ALA fluorescence, combined intraoperative strategies and correlation with survival. J Neurosurg Sci. https://doi.org/10.23736/S0390-5616.19.04787-8
Altieri R et al (2022) Anatomical distribution of cancer stem cells between enhancing nodule and FLAIR hyperintensity in supratentorial glioblastoma: time to recalibrate the surgical target? Neurosurg Rev. https://doi.org/10.1007/s10143-022-01863-8
McMahon DJ, Gleeson JP, O’Reilly S, Bambury RM (2022) Management of newly diagnosed glioblastoma multiforme: current state of the art and emerging therapeutic approaches. Med Oncol 39:129. https://doi.org/10.1007/s12032-022-01708-w
Wu A et al (2022) Trends and outcomes of early and late palliative care consultation for adult patients with glioblastoma: a SEER-medicare retrospective study. Neurooncol Pract 9:299–309. https://doi.org/10.1093/nop/npac026
Giammalva GR et al (2018) End-of-life care in high-grade glioma patients. The palliative and supportive perspective. Brain Sci 8:125. https://doi.org/10.3390/brainsci8070125
Jiang Y et al (2022) CircLRFN5 inhibits the progression of glioblastoma via PRRX2/GCH1 mediated ferroptosis. J Exp Clin Cancer Res 41:307. https://doi.org/10.1186/s13046-022-02518-8
Kim H-J et al (2022) Blood monocyte-derived CD169+ macrophages contribute to antitumor immunity against glioblastoma. Nat Commun 13:6211. https://doi.org/10.1038/s41467-022-34001-5
Bonosi L et al (2022) Liquid biopsy in diagnosis and prognosis of high-grade gliomas; state-of-the-art and literature review. Life 12:407. https://doi.org/10.3390/life12030407
Choi S, Yin J (2022) Prospective approaches to enhancing CAR T cell therapy for glioblastoma. Front Immunol. https://doi.org/10.3389/fimmu.2022.1008751
Qian L, Mao L, Mo W, Wang R, Zhang Y (2022) Resveratrol enhances the radiosensitivity by inducing DNA damage and antitumor immunity in a glioblastoma Rat model under 3 T MRI monitoring. J Oncol 2022:1–13. https://doi.org/10.1155/2022/9672773
Hersh AM et al (2022) Applications of focused ultrasound for the treatment of glioblastoma: a new frontier. Cancers (Basel) 14:4920. https://doi.org/10.3390/cancers14194920
Toccaceli G, Barbagallo G, Peschillo S (2019) Low-intensity focused ultrasound for the treatment of brain diseases: safety and feasibility. Theranostics 9:537–539. https://doi.org/10.7150/thno.31765
Bunevicius A, Pikis S, Padilla F, Prada F, Sheehan J (2022) Sonodynamic therapy for gliomas. J Neurooncol 156:1–10. https://doi.org/10.1007/s11060-021-03807-6
Bilmin K, Kujawska T, Grieb P (2019) Sonodynamic therapy for gliomas. Perspectives and prospects of selective sonosensitization of glioma cells. Cells 8:1428. https://doi.org/10.3390/cells8111428
Tutak I, Ozdil B, Uysal A (2022) Voxtalisib and low intensity pulsed ultrasound combinatorial effect on glioblastoma multiforme cancer stem cells via PI3K/AKT/MTOR. Pathol Res Pract 239:154145. https://doi.org/10.1016/j.prp.2022.154145
D’Ammando A et al (2021) Sonodynamic therapy for the treatment of intracranial gliomas. J Clin Med 10:1101. https://doi.org/10.3390/jcm10051101
Moon H et al (2022) Enhanced delivery to brain using sonosensitive liposome and microbubble with focused ultrasound. Biomater Adv 141:213102. https://doi.org/10.1016/j.bioadv.2022.213102
Page MJ et al (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. https://doi.org/10.1136/bmj.n71
Hayashi S et al (2009) Mechanism of photofrin-enhanced ultrasound-induced human glioma cell death. Anticancer Res 29:897–905 (PMID: 19414325)
Xu Z-Y et al (2012) Glioma stem-like cells are less susceptible than glioma cells to sonodynamic therapy with photofrin. Technol Cancer Res Treat 11:615–623. https://doi.org/10.7785/tcrt.2012.500277
Hao D, Song Y, Che Z, Liu Q (2014) Calcium overload and in vitro apoptosis of the C6 glioma cells mediated by sonodynamic therapy (hematoporphyrin monomethyl ether and ultrasound). Cell Biochem Biophys 70:1445–1452. https://doi.org/10.1007/s12013-014-0081-7
Gonzales J, Nair RK, Madsen SJ, Krasieva T, Hirschberg H (2016) Focused ultrasound-mediated sonochemical internalization: an alternative to light-based therapies. J Biomed Opt 21:078002. https://doi.org/10.1117/1.JBO.21.7.078002
Chen L et al (2017) Combination of sonodynamic with temozolomide inhibits C6 glioma migration and promotes mitochondrial pathway apoptosis via suppressing NHE-1 expression. Ultrason Sonochem 39:654–661. https://doi.org/10.1016/j.ultsonch.2017.05.013
Sun Y et al (2018) Sonodynamic therapy induces oxidative stress, DNA damage and apoptosis in glioma cells. RSC Adv 8:36245–36256. https://doi.org/10.1039/C8RA07099G
Dai S, Hu S, Wu C (2009) Apoptotic effect of sonodynamic therapy mediated by hematoporphyrin monomethyl ether on C6 glioma cells in vitro. Acta Neurochir (Wien) 151:1655–1661. https://doi.org/10.1007/s00701-009-0456-5
Sheehan K et al (2020) Investigation of the tumoricidal effects of sonodynamic therapy in malignant glioblastoma brain tumors. J Neurooncol. https://doi.org/10.1007/s11060-020-03504-w
Shono K et al (2021) Elevated cellular PpIX potentiates sonodynamic therapy in a mouse glioma stem cell-bearing glioma model by downregulating the Akt/NF-ΚB/MDR1 pathway. Sci Rep 11:15105. https://doi.org/10.1038/s41598-021-93896-0
Shen Y et al (2021) An in vitro study on the antitumor effect of sonodynamic therapy using sinoporphyrin sodium on human glioblastoma cells. Ultrasonics 110:106272. https://doi.org/10.1016/j.ultras.2020.106272
Nonaka M et al (2009) Sonodynamic therapy consisting of focused ultrasound and a photosensitizer causes a selective antitumor effect in a Rat intracranial glioma model. Anticancer Res 29:943–950 (PMID: 19414331)
Ohmura T et al (2011) Sonodynamic therapy with 5-aminolevulinic acid and focused ultrasound for deep-seated intracranial glioma in Rat. Anticancer Res 31:2527–2533 (PMID: 21873170)
Jeong E-J et al (2012) Sonodynamically induced antitumor effects of 5-aminolevulinic acid and fractionated ultrasound irradiation in an orthotopic Rat glioma model. Ultrasound Med Biol 38:2143–2150. https://doi.org/10.1016/j.ultrasmedbio.2012.07.026
Tserkovsky DA, Alexandrova EN, Chalau VN, Istomin YP (2012) Effects of combined sonodynamic and photodynamic therapies with photolon on a glioma C6 tumor model. Exp Oncol 34:332–335 (PMID: 23302991)
Song D et al (2014) Study of the mechanism of sonodynamic therapy in a Rat glioma model. Onco Targets Ther. https://doi.org/10.2147/OTT.S52426
Ju D et al (2016) Hyperthermotherapy enhances antitumor effect of 5-aminolevulinic acid-mediated sonodynamic therapy with activation of caspase-dependent apoptotic pathway in human glioma. Tumor Biol 37:10415–10426. https://doi.org/10.1007/s13277-016-4931-3
Suehiro S et al (2018) Enhancement of antitumor activity by using 5-ALA–mediated sonodynamic therapy to induce apoptosis in malignant gliomas: significance of high-intensity focused ultrasound on 5-ALA-SDT in a mouse glioma model. J Neurosurg 129:1416–1428. https://doi.org/10.3171/2017.6.JNS162398
Pi Z et al (2019) Sonodynamic therapy on intracranial glioblastoma xenografts using sinoporphyrin sodium delivered by ultrasound with microbubbles. Ann Biomed Eng 47:549–562. https://doi.org/10.1007/s10439-018-02141-9
Sun Y et al (2019) Tumor targeting DVDMS-nanoliposomes for an enhanced sonodynamic therapy of gliomas. Biomater Sci 7:985–994. https://doi.org/10.1039/C8BM01187G
Yoshida M et al (2019) Sonodynamic therapy for malignant glioma using 220-KHz transcranial magnetic resonance imaging-guided focused ultrasound and 5-aminolevulinic acid. Ultrasound Med Biol 45:526–538. https://doi.org/10.1016/j.ultrasmedbio.2018.10.016
An Y et al (2020) Sinoporphyrin sodium is a promising sensitizer for photodynamic and sonodynamic therapy in glioma. Oncol Rep. https://doi.org/10.3892/or.2020.7695
Prada F et al (2020) Fluorescein-mediated sonodynamic therapy in a Rat Glioma model. J Neurooncol 148:445–454. https://doi.org/10.1007/s11060-020-03536-2
Raspagliesi L et al (2021) Intracranial sonodynamic therapy with 5-aminolevulinic acid and sodium fluorescein: safety study in a porcine model. Front Oncol. https://doi.org/10.3389/fonc.2021.679989
Shergalis A, Bankhead A, Luesakul U, Muangsin N, Neamati N (2018) Current challenges and opportunities in treating glioblastoma. Pharmacol Rev 70:412–445. https://doi.org/10.1124/pr.117.014944
Biserova K, Jakovlevs A, Uljanovs R, Strumfa I (2021) Cancer stem cells: significance in origin, pathogenesis and treatment of glioblastoma. Cells 10:621. https://doi.org/10.3390/cells10030621
Xu L et al (2021) Topography of transcriptionally active chromatin in glioblastoma. Sci Adv. https://doi.org/10.1126/sciadv.abd4676
De Luca C et al (2022) Regional development of glioblastoma: the anatomical conundrum of cancer biology and its surgical implication. Cells 11:1349. https://doi.org/10.3390/cells11081349
Bush NAO, Chang SM, Berger MS (2017) Current and future strategies for treatment of glioma. Neurosurg Rev 40:1–14. https://doi.org/10.1007/s10143-016-0709-8
Gerritsen JKW et al (2022) Safe surgery for glioblastoma: recent advances and modern challenges. Neurooncol Pract 9:364–379. https://doi.org/10.1093/nop/npac019
Tan AC et al (2020) Management of glioblastoma: state of the art and future directions. CA Cancer J Clin 70:299–312. https://doi.org/10.3322/caac.21613
Evangelista L, Mansi L (2022) Theragnostics applications and challenges. Q J Nucl Med Mol Imaging. https://doi.org/10.23736/S1824-4785.21.03439-7
Landgraf L et al (2022) Focused ultrasound treatment of a spheroid in vitro tumour model. Cells 11:1518. https://doi.org/10.3390/cells11091518
Coluccia D et al (2014) First noninvasive thermal ablation of a brain tumor with MR-guided focusedultrasound. J Ther Ultrasound 2:17. https://doi.org/10.1186/2050-5736-2-17
Wang S, Zderic V, Frenkel V (2010) Extracorporeal, low-energy focused ultrasound for noninvasive and nondestructive targeted hyperthermia. Fut Oncol 6:1497–1511. https://doi.org/10.2217/fon.10.101
Sawyers CL (2008) The cancer biomarker problem. Nature 452:548–552. https://doi.org/10.1038/nature06913
Burgess A et al (2014) Alzheimer disease in a mouse model: MR imaging–guided focused ultrasound targeted to the hippocampus opens the blood-brain barrier and improves pathologic abnormalities and behavior. Radiology 273:736–745. https://doi.org/10.1148/radiol.14140245
Tsai S-J (2015) Transcranial focused ultrasound as a possible treatment for major depression. Med Hypotheses 84:381–383. https://doi.org/10.1016/j.mehy.2015.01.030
Hynynen K et al (2006) Focal disruption of the blood–brain barrier due to 260-KHz ultrasound bursts: a method for molecular imaging and targeted drug delivery. J Neurosurg 105:445–454. https://doi.org/10.3171/jns.2006.105.3.445
Cohen-Inbar O, Xu Z, Sheehan JP (2016) Focused ultrasound-aided immunomodulation in glioblastoma multiforme: a therapeutic concept. J Ther Ultrasound 4:2. https://doi.org/10.1186/s40349-016-0046-y
Gong Z, Dai Z (2021) Design and challenges of sonodynamic therapy system for cancer theranostics: from equipment to sensitizers. Adv Sci 8:2002178. https://doi.org/10.1002/advs.202002178
McHale AP, Callan JF, Nomikou N, Fowley C, Callan B (2016) Sonodynamic therapy: concept, mechanism and application to cancer treatment. Adv Exp Med Biol 880:429–50. https://doi.org/10.1007/978-3-319-22536-4_22
Ding C, Xing D (2005). Studies on the sonosensitization mechanism of ultrasound with ATX-70 in sonodynamic therapy. In: Proc. SPIE 5630, optics in health care and biomedical optics: diagnostics and treatment II (18 January 2005). https://doi.org/10.1117/12.572824
Wu S-K, Santos MA, Marcus SL, Hynynen K (2019) MR-guided focused ultrasound facilitates sonodynamic therapy with 5-aminolevulinic acid in a Rat glioma model. Sci Rep 9:10465. https://doi.org/10.1038/s41598-019-46832-2
Zhang C et al (2022) A novel hypocrellin-based assembly for sonodynamic therapy against glioblastoma. J Mater Chem B 10:57–63. https://doi.org/10.1039/D1TB01886H
Roberts JW, Powlovich L, Sheybani N, LeBlang S (2022) Focused ultrasound for the treatment of glioblastoma. J Neurooncol 157:237–247. https://doi.org/10.1007/s11060-022-03974-0
Pan X et al (2018) Sonodynamic therapy (SDT): a novel strategy for cancer nanotheranostics. Sci China Life Sci 61:415–426. https://doi.org/10.1007/s11427-017-9262-x
Sheehan K et al (2020) Investigation of the tumoricidal effects of sonodynamic therapy in malignant glioblastoma brain tumors. J Neurooncol 148:9–16. https://doi.org/10.1007/s11060-020-03504-w
Abdolhosseinzadeh A, Mojra A, Ashrafizadeh AA (2019) Numerical study on thermal ablation of brain tumor with intraoperative focused ultrasound. J Therm Biol 83:119–133. https://doi.org/10.1016/j.jtherbio.2019.05.019
Zhang X et al (2021) Focused ultrasound radiosensitizes human cancer cells by enhancement of DNA damage. Strahlenther Onkol 197:730–743. https://doi.org/10.1007/s00066-021-01774-5
Hu S et al (2020) Focused ultrasound-induced cavitation sensitizes cancer cells to radiation therapy and hyperthermia. Cells 9:2595. https://doi.org/10.3390/cells9122595
Arvanitis CD, Vykhodtseva N, Jolesz F, Livingstone M, McDannold N (2016) Cavitation-enhanced nonthermal ablation in deep brain targets: feasibility in a large animal model. J Neurosurg 124:1450–1459. https://doi.org/10.3171/2015.4.JNS142862
Jones RM, McMahon D, Hynynen K (2020) Ultrafast three-dimensional microbubble imaging in vivo predicts tissue damage volume distributions during nonthermal brain ablation. Theranostics 10:7211–7230. https://doi.org/10.7150/thno.47281
McDannold N, Clement GT, Black P, Jolesz F, Hynynen K (2010) Transcranial magnetic resonance imaging– guided focused ultrasound surgery of brain tumors. Neurosurgery 66:323–332. https://doi.org/10.1227/01.NEU.0000360379.95800.2F
Liu Q, Wang X, Wang P, Xiao L, Hao Q (2007) Comparison between sonodynamic effect with protoporphyrin IX and hematoporphyrin on sarcoma 180. Cancer Chemother Pharmacol 60:671–680. https://doi.org/10.1007/s00280-006-0413-4
Nomikou N, Li YS, McHale AP (2010) Ultrasound-enhanced drug dispersion through solid tumours and its possible role in aiding ultrasound-targeted cancer chemotherapy. Cancer Lett 288:94–98. https://doi.org/10.1016/j.canlet.2009.06.028
Son S et al (2020) Multifunctional sonosensitizers in sonodynamic cancer therapy. Chem Soc Rev 49:3244–3261. https://doi.org/10.1039/C9CS00648F
Wang C, Tian Y, Wu B, Cheng W (2022) Recent progress toward imaging application of multifunction sonosensitizers in sonodynamic therapy. Int J Nanomed 17:3511–3529. https://doi.org/10.2147/IJN.S370767
Tian Y et al (2013) Effects of 5-aminolevulinic acid-mediated sonodynamic therapy on macrophages. Int J Nanomed. https://doi.org/10.2147/IJN.S39844
Ram Z et al (2006) Magnetic resonance imaging-guided, high-intensity focused ultrasound for brain tumor therapy. Neurosurgery 59:949–956. https://doi.org/10.1227/01.NEU.0000254439.02736.D8
Cohen ZR et al (2007) Magnetic resonance imaging-guided focused ultrasound for thermal ablation in the brain. Neurosurgery 60:593–600. https://doi.org/10.1227/01.NEU.0000245606.99946.C6
Pirkkala L, Nykänen P, Sistonen L (2001) Roles of the heat shock transcription factors in regulation of the heat shock response and beyond. FASEB J 15:1118–1131. https://doi.org/10.1096/fj00-0294rev
Hildebrandt B (2022) The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 43:33–56. https://doi.org/10.1016/S1040-8428(01)00179-2
Cippitelli M et al (2005) Hyperthermia enhances CD95-ligand gene expression in T lymphocytes. J Immunol 174:223–232. https://doi.org/10.4049/jimmunol.174.1.223
Yumita N, Han Q-S, Kitazumi I, Umemura S (2007) Sonodynamically-induced apoptosis, necrosis, and active oxygen generation by mono-l-aspartyl chlorin E6. Cancer Sci. https://doi.org/10.1111/j.1349-7006.2007.00653.x
Su X et al (2015) Sonodynamic therapy induces the interplay between apoptosis and autophagy in K562 cells through ROS. Int J Biochem Cell Biol 60:82–92. https://doi.org/10.1016/j.biocel.2014.12.023
Wan Q et al (2019) Imaging-guided focused ultrasound-induced thermal and sonodynamic effects of nanosonosensitizers for synergistic enhancement of glioblastoma therapy. Biomater Sci 7:3007–3015. https://doi.org/10.1039/C9BM00292H
Byun K-T, Kim KY, Kwak H-Y (2005) Sonoluminescence characteristics from micron and submicron bubbles. J Kor Phys Soc 47(6):1010–1022
Guo J, Pan X, Wang C, Liu H (2022) Molecular imaging-guided sonodynamic therapy. Bioconjug Chem 33:993–1010. https://doi.org/10.1021/acs.bioconjchem.1c00288
Hiraoka W, Honda H, Feril LB, Kudo N, Kondo T (2006) Comparison between sonodynamic effect and photodynamic effect with photosensitizers on free radical formation and cell killing. Ultrason Sonochem 13:535–542. https://doi.org/10.1016/j.ultsonch.2005.10.001
Duco W, Grosso V, Zaccari D, Soltermann AT (2016) Generation of ROS mediated by mechanical waves (ultrasound) and its possible applications. Methods 109:141–148. https://doi.org/10.1016/j.ymeth.2016.07.015
Choi V, Rajora MA, Zheng G (2020) Activating drugs with sound: mechanisms behind sonodynamic therapy and the role of nanomedicine. Bioconjug Chem 31:967–989. https://doi.org/10.1021/acs.bioconjchem.0c00029
Canaparo R, Foglietta F, Barbero N, Serpe L (2022) The promising interplay between sonodynamic therapy and nanomedicine. Adv Drug Deliv Rev 189:114495. https://doi.org/10.1016/j.addr.2022.114495
Rosenthal I, Sostaric JZ, Riesz P (2004) Sonodynamic therapy––a review of the synergistic effects of drugs and ultrasound. Ultrason Sonochem 11:349–363. https://doi.org/10.1016/j.ultsonch.2004.03.004
Lafond M, Yoshizawa S, Umemura S (2019) Sonodynamic therapy: advances and challenges in clinical translation. J Ultrasound Med 38:567–580. https://doi.org/10.1002/jum.14733
Gong F, Cheng L, Yang N, Betzer O, Feng L, Zhou Q, Li Y, Chen R, Popovtzer R, Liu Z (2019) Ultrasmall oxygen-deficient bimetallic oxide MnWO X nanoparticles for depletion of endogenous GSH and enhanced sonodynamic cancer therapy. Adv Mater 31:1900730. https://doi.org/10.1002/adma.201900730
Zhang P, Ren Z, Chen Z, Zhu J, Liang J, Liao R, Wen J (2018) Iron oxide nanoparticles as nanocarriers to improve chlorin E6-based sonosensitivity in sonodynamic therapy. Drug Des Dev Ther 12:4207–4216. https://doi.org/10.2147/DDDT.S184679
Jia Y, Wang X, Liu Q, Leung AW, Wang P, Xu C (2017) Sonodynamic action of hypocrellin B triggers cell apoptoisis of breast cancer cells involving caspase pathway. Ultrasonics 73:154–161. https://doi.org/10.1016/j.ultras.2016.09.013
Osaki T, Ono M, Uto Y, Ishizuka M, Tanaka T, Yamanaka N, Kurahashi T, Azuma K, Murahata Y, Tsuka T et al (2016) Sonodynamic therapy using 5-aminolevulinic acid enhances the efficacy of bleomycin. Ultrasonics 67:76–84. https://doi.org/10.1016/j.ultras.2016.01.003
Moosavi Nejad S, Takahashi H, Hosseini H, Watanabe A, Endo H, Narihira K, Kikuta T, Tachibana K (2016) Acute effects of sono-activated photocatalytic titanium dioxide nanoparticles on oral squamous cell carcinoma. Ultrason Sonochem 32:95–101. https://doi.org/10.1016/j.ultsonch.2016.02.026
Xu H, Sun X, Yao J, Zhang J, Zhang Y, Chen H, Dan J, Tian Z, Tian Y (2015) The decomposition of protoporphyrin IX by ultrasound is dependent on the generation of hydroxyl radicals. Ultrason Sonochem 27:623–630. https://doi.org/10.1016/j.ultsonch.2015.04.024
Araújo Martins Y, Zeferino Pavan T, Fonseca Vianna Lopez R (2021) Sonodynamic therapy: ultrasound parameters and in vitro experimental configurations. Int J Pharm 610:121243. https://doi.org/10.1016/j.ijpharm.2021.121243
Smits GAHJ, Oosterhof GON, de Ruyter AE, Schalken JA, Debruyne FMJ (1991) Cytotoxic effects of high energy shock waves in different in vitro models: influence of the experimental set-up. J Urol 145:171–175. https://doi.org/10.1016/S0022-5347(17)38284-8
Zhang Q, Bao C, Cai X, Jin L, Sun L, Lang Y, Li L (2018) Sonodynamic therapy-assisted immunotherapy: a novel modality for cancer treatment. Cancer Sci 109:1330–1345. https://doi.org/10.1111/cas.13578
Debela DT et al (2021) New approaches and procedures for cancer treatment: current perspectives. SAGE Open Med 9:205031212110343. https://doi.org/10.1177/20503121211034366
Gao Z et al (2013) Sonodynamic therapy inhibits angiogenesis and tumor growth in a Xenograft Mouse Model. Cancer Lett 335:93–99. https://doi.org/10.1016/j.canlet.2013.02.006.
Jameel A, Bain P, Nandi D, Jones B, Gedroyc W (2021) Device profile of exAblate Neuro 4000, the leading system for brain magnetic resonance guided focused ultrasound technology: an overview of its safety and efficacy in the treatment of medically refractory essential tremor. Expert Rev Med Dev 18:429–437
Focused Ultrasound Foundation – State of the field 2022
Funding
Open access funding provided by Università degli Studi di Palermo within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Study design: LB, SM (Silvia Marino); Manuscript writing: SM (Sofia Musso), KG, UEB, FB and LB studies selecting: KG, UEB; data analysis: SM (Sofia Musso), KG; study quality evaluating: UEB, LB; manuscript revising: RG, LB (Lara Brunasso), RC; Project Administrators: LB, DGI, RM. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bonosi, L., Marino, S., Benigno, U.E. et al. Sonodynamic therapy and magnetic resonance-guided focused ultrasound: new therapeutic strategy in glioblastoma. J Neurooncol 163, 219–238 (2023). https://doi.org/10.1007/s11060-023-04333-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04333-3