Abstract
Purpose
Lower-grade glioma (LGG) is rare among patients above the age of 60 (“elderly”). Previous studies reported poor outcome, likely due to the inclusion of isocitrate dehydrogenase (IDH) wildtype astrocytomas and advocated defensive surgical and adjuvant treatment. This study set out to question this paradigm analyzing a contemporary cohort of patients with IDH mutant astrocytoma and oligodendroglioma WHO grade 2 and 3.
Methods
Elderly patients treated in our department for a supratentorial, hemispheric LGG between 2009 and 2019 were retrospectively analyzed for patient-, tumor- and treatment-related factors and progression-free survival (PFS) and compared to patients aged under 60. Inclusion required the availability of subtype-defining molecular data and pre- and post-operative tumor volumes.
Results
207 patients were included, among those 21 elderlies (10%). PFS was comparable between elderly and younger patients (46 vs. 54 months; p = 0.634). Oligodendroglioma was more common in the elderly (76% vs. 46%; p = 0.011). Most patients underwent tumor resection (elderly: 81% vs. younger: 91%; p = 0.246) yielding comparable residual tumor volumes (elderly: 7.8 cm3; younger: 4.1 cm3; p = 0.137). Adjuvant treatment was administered in 76% of elderly and 61% of younger patients (p = 0.163). Uni- and multi-variate survival analyses identified a tumor crossing the midline, surgical strategy, and pre- and post-operative tumor volumes as prognostic factors.
Conclusion
Elderly patients constitute a small fraction of molecularly characterized LGGs. In contrast to previous reports, favorable surgical and survival outcomes were achieved in our series comparable to those of younger patients. Thus, intensified treatment including maximal safe resection should be advocated in elderly patients whenever feasible.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lower-grade gliomas (LGG) are primary brain tumors that share mutations in the isocitrate dehydrogenase gene (IDHmut) [1]. They are classified by the World Health Organization (WHO) as grade 2 and 3 tumors. Compared to their wildtype counterpart (IDHwt), which are classified as glioblastoma IDHwt [2], IDHmut astrocytomas fare much better, although malignant transformation into grade 4 tumors is not uncommon [3]. Another important feature of LGG is the young age at first diagnosis. The median age of IDHmut astrocytoma and IDHmut, 1p19q co-deleted oligodendroglioma patients is 38.1 and 45.4 years, respectively [4]. As age has univocally been shown to be an important prognostic factor, it has guided treatment decisions [5,6,7,8]. An age below 40 years along with other favorable factors is considered a positive prognostic factor and a watch and wait strategy may be pursued after resection [5]. In contrast, in patients over 40, upfront maximal safe resection followed by adjuvant chemoradiation is recommended. In 2016, the WHO classification has introduced an important shift from a pure histological towards an integrated molecular diagnosis creating a new paucity in the rare elderly LGG patient population [2, 9]. Previous studies, not stratified for IDH mutation status or other molecular markers, have tried to elucidate optimal treatment and patient outcome but most likely included IDHwt glioblastomas (formerly astrocytoma IDHwt) which are much more common in the elderly than IDHmut glioma and show a dismal prognosis [10]. This may have masked positive treatment effects in previous analyses. Conclusions as to restrain from maximized surgery or adjuvant treatment are therefore questionable [11]. In lack of a molecularly defined elderly LGG cohort, treatment recommendations cannot be adequately given. Of note, there is no clear age cut-off defining “elderly” patients in the context of LGG. The available literature shows a wide range starting from 50 years (50–60 years) [11,12,13,14,15]. In this study, we chose the most conservative age cut-off of 60 years to establish a representative elderly cohort. Taken together, this study aims to analyze surgical and survival outcomes in a molecularly characterized, purely IDHmut cohort of elderly lower-grade glioma patients.
Materials and methods
Patient selection
A database search was conducted for lower-grade glioma patients who were treated in our neurosurgical department from 2009 to 2019 (Fig. 1). Surgery included stereotactic or open biopsy, partial, subtotal and gross total resection. Use of surgical adjuncts such as intraoperative MRI (iMRI), 5-aminolaevulinic acid (5-ALA) and intraoperative neuromonitoring (IONM) were also recorded. For all patients, medical records were reviewed for clinical information (Karnofsky performance status (KPS), neurological symptoms). Approval from the ethics committee of the Medical Faculty of the University of Heidelberg was obtained before initiation of the study and patient consent was waived (reference S-005/2003, as of 31.01.2003).
Flowchart depicting patient selection of the lower-grade glioma cohort. After applying the inclusion and exclusion criteria on 322 identified LGG patients, 207 patients were left for the final analysis. 186 patients were considered “younger” and 21 “elderly” based on the pre-specified age cut-off of 60 years
Histopathologic and molecular diagnosis
Neuropathologic diagnostics was performed in concordance with the WHO classification from 2016 [9]. The following molecular data were obtained as part of daily routine: IDH mutation status was obtained by immunohistochemistry or direct sequencing of the mutation hotspot region for all patients [16]. 1p/19q codeletion status was available for all patients. Genome wide methylation analysis with copy number analysis was available for 171 patients (83%) and was generated using the Illumina HumanMethylation450 (450 k) or Methylation EPIC (850 k) array platforms as described [17].
MRI evaluation
MRI was available at initial diagnosis until a few days prior to surgery (“preoperative”). Imaging sequences included T1-, T1 + gadolinium, T2- and fluid-attenuated inversion-recovery (FLAIR)-weighted sequences. Manual segmentation was performed using the Brainlab™ software (Brainlab, Germany) to quantify tumor volumes on T1- contrast-enhanced (CE) and FLAIR-weighted images in cm3 at pre- and early (< 72 h) post-operative scans. Tumor features such as eloquent location, midline crossing and involvement of the subventricular zone (SVZ) were also evaluated.
“The Cancer Genome Atlas” (TCGA) data analysis
The combined glioblastoma (GBM) and LGG cohort was analyzed using the publicly available cBioPortal platform. Only IDHmut WHO grade 2 and 3 tumors were considered.
Statistical analysis
Statistical analyses were performed using the SPSS statistics software (IBM, version 28.0.0.0) and Prism (GraphPad, version 9.4.1). Comparative statistical tests comprised Fisher’s exact test and Chi square test for categorial and Mann–Whitney-U test for continuous variables. For survival analysis, progression-free survival (PFS) was defined by the time interval from first surgery to first radiological progression, last follow-up or death, and cancer-related survival from first surgery until last follow-up or tumor-related death. A univariate log-rank test was used to identify patient-, tumor- and treatment-related variables with prognostic impact. For multivariate analysis, a Cox proportional hazard model was used with inclusion of all covariates found to be significant in univariate analysis (p < 0.05) by stepwise backward selection. Only cases with all covariates available were considered for multivariate analysis (n = 167).
Results
Baseline characteristics of the molecularly characterized patient cohort
Patients treated in our department from 2009 to 2019 were screened for a supratentorial astrocytoma or oligodendroglioma WHO grade 2 or 3 (Fig. 1). 322 consecutive patients were identified and 19 excluded (IDHwt astrocytoma). Considering only patients with first surgery in our department and MRI data sets allowing volumetric analysis, the final study cohort comprised 207 patients. The median age of the study cohort was 41 years (range 17–79). 21/207 patients were over the age of 60 and were considered elderly, constituting 10% of all LGG patients (Fig. 2a). Oligodendroglioma patients were older than astrocytoma patients (36 vs. 44 years; p = 0.0002, Fig. 2b). Baseline patient characteristics were comparable between younger and elderly patients except for the KPS which was significantly higher in younger patients (median KPS 95 vs. 87; p < 0.001; Table 1). However, there were significant differences in tumor-related factors (Table 1). SVZ involvement was significantly more frequent in elderly (15/21 = 71%) than in younger patients (84/186 = 45%; p = 0.036). Also, oligodendroglioma was significantly overrepresented in the elderly (16/21 = 76% vs. 86/186 = 46%; p = 0.011; Fig. 2c) whereas WHO grade did not show a significant difference between the two groups (p = 0.105; Fig. 2c). The rates of tumors with contrast enhancement and those crossing the midline as well as preoperative tumor volumes (FLAIR and CE) were comparable as well (Table 1, Fig. 2g).
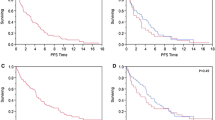
a Distribution of the study cohort. 21 patients (10%) were above the age of 60 and were considered elderly. Median age of the total population was 41 years (range 17–79). b Oligodendroglioma patients were older than astrocytoma patients (36 vs. 44 years, p < 0.001). c Oligodendroglioma was significantly overrepresented in the elderly (76 vs. 54%; p = 0.011), whereas WHO grade was not (p = 0.105). d, e WHO grade prognosticated PFS only in oligodendroglioma patients. f Surgical treatment strategies were comparable in the elderly and the younger with most patients receiving tumor resection in both cohorts (81% vs. 91%, p = 0.246). Adjuvant treatment was administered in 61% of the younger and 76% of the elderly patients (p = 0.163). g, h Preoperative and residual FLAIR tumor volumes did not differ between both age groups (p = 0.223, p = 0.137). i Adjuvant treatment consisted of radiotherapy or chemotherapy alone or chemoradiation and was dependent on tumor subtype. *p < 0.05, ***p < 0.001
Surgical and adjuvant treatment strategies in elderly and younger patients
Surgical results were comparable in both age groups. The rates of patients undergoing resection (vs. biopsy) did not differ statistically in younger (91%) and elderly (81%) patients (p = 0.246; Fig. 2f). There was a trend towards a more eager use of surgical adjuncts (iMRI, IONM, awake craniotomy; Table 1) in younger patients, nonetheless yielding comparable residual volumes (median FLAIR volume 7.8 cm3 (elderly) vs. 4.1 cm3 (younger); p = 0.137; Fig. 2h). 34/207 patients had near complete tumor resection with residual tumor volumes < 1 cm3, of which 33 were younger (18%) and one elderly (5%) (p = 0.150). Importantly, the rates of postoperative neurological deterioration or revision surgery did not differ between the two groups (p = 0.111 and p = 0.658, respectively). Also, there was no significant difference in the administration of adjuvant treatment (76% elderly vs. 61% younger; p = 0.163). 39% of the younger patients did not receive any adjuvant treatment, mainly because of a favorable risk profile (e.g. extended resections) (Fig. 2f). In the elderly, reasoning for not administering adjuvant treatment was mixed. Of the 5 elderly patients who did not receive adjuvant treatment (24%), 3 had a favorable risk profile (oligodendroglioma WHO grade 2, gross total resection), 1 patient refused further treatment (astrocytoma WHO grade 3) and 1 patient was simultaneously diagnosed with an acute myeloid leukemia and died of its complications (astrocytoma WHO grade 3). Treatment regimens were heterogeneous and mainly dependent on tumor subtype and WHO grade. 70% of the younger oligodendroglioma WHO grade 2 patients did not receive adjuvant treatment in contrast to the elderly, where adjuvant treatment was administered in 60%. Similarly, for astrocytoma WHO grade 2 patients, 62% of the younger cohort did not receive adjuvant treatment as opposed to the elderly where all three patients received radiotherapy (n = 1) or chemotherapy (n = 2). In WHO grade 3 patients, a watch-and-wait strategy was not favored in both cohorts and only pursued in individual cases. In oligodendroglioma WHO grade 3, chemoradiation was the most frequently administered treatment regimen (younger: 70%, elderly: 63%) whereas in astrocytoma WHO grade 3 tumors only younger patients received combined treatment. For the two elderly astrocytoma WHO grade 3 patients, chemotherapy alone was administered (Fig. 2i).
Identification of prognostic factors
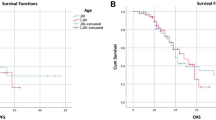
Next, we sought to describe patient outcome and identify prognostic factors. Within a median follow-up of 54 months in elderly and 70 months in younger patients, tumor progression occurred in 57% and 55% of patients, respectively (p = 0.497). Death (due to any reason) occurred in 24% of elderly and 9% of younger patients (p = 0.02) resulting in inferior overall survival (OS) in the elderly cohort (Table 1, Suppl. Fig. 1a). However, of the 5 deceased elderly patients, 3 were not tumor-related deaths whereas 15 out of 17 younger patients died of tumor-related reasons. After censoring non-cancer-related deaths, cancer-related survival between the two cohorts did not differ (p = 0.638; Fig. 3b). For further survival analysis, we focused on PFS since time to progression and subsequent re-exposition of treatment is highly relevant in slowly progressing tumors. Importantly, PFS was comparable in elderly and younger patients (median 46 vs. 54 months; p = 0.792; Fig. 3a). Subsequent univariate analysis of all patient-, tumor- and treatment-related factors was performed for the complete cohort, and several factors were identified. Midline crossing conferred shorter PFS (median 31 vs. 59 months; p < 0.0001; Fig. 3c). Also, preoperative tumor volumes larger than the corresponding median volume (FLAIR: 41 cm3, CE: 0 cm3) were associated with inferior PFS (FLAIR: median 41 vs. 61 months, p = 0.01; CE: median 45 vs. 57 months; p = 0.034; Fig. 3d and e). Concerning treatment-related factors, patients with tumor resection had a significantly longer PFS than patients with biopsy only (57 vs. 24 months; p = 004; Fig. 3e). Moreover, residual tumor volumes smaller than the individual median volume (FLAIR: 4.8 cm3; CE: 0 cm3) were linked to superior PFS (FLAIR: 61 vs. 41 months, p = 0.01; CE: 61 vs. 32 months, p = 0.016). To rule out that these results were biased by patient age, we performed subgroup analysis (elderly vs. younger patients) for every individual prognostic factor, but results coincided (Suppl. Fig. 2). Adjuvant treatment and tumor subtype did not impact PFS (Table 2). Within the tumor subtypes, WHO grade (2 vs. 3) was only prognostic in oligodendroglioma patients (median PFS 59 vs. 32 months, p = 0.0274, Fig. 2d and e). Subsequent multivariate analysis with inclusion of all variables significant in univariate analysis revealed “midline crossing” as the only independent prognostic factor negatively associated with PFS (HR 2.315; 95% CI 1.39–3.85; p = 0.001; Table 2).
Kaplan–Meier curves depicting patient-, tumor- and treatment-related factors. Univariate analysis identified midline crossing, surgical strategy, and pre- and post-operative tumor volumes (FLAIR and CE) as prognosticators for PFS. For continuous variables (pre- and post-operative tumor volumes), the corresponding median volumes were used for dichotomization
Comparison with “The Cancer Genome Atlas” (TCGA) IDH mutant study cohort
To put our findings into perspective, survival data of the combined GBM and LGG cohort of TCGA was extracted [4, 18]. Only patients with a connotated age and a confirmed WHO grade 2 or 3 tumor with IDH1 (n = 358; 51%) or IDH2 (n = 19; 2.6%) mutations were included (n = 377). As in our cohort, 10% (n = 37) of patients in the TCGA were above the age of 60. Age-dependent distribution of tumor subtypes and WHO grade coincide well with our findings (Suppl. Fig. 3). Only OS, but not cancer-related survival was available for the TCGA cohort and was shorter than in our cohort (Suppl. Fig. 2a). Nevertheless, similar to our cohort, OS was inferior in elderly patients compared to younger ones (52 vs. 96 months; p < 0.0001). When looking at the tumor subtypes, OS differed among WHO grade 2 and 3 tumors in oligodendroglioma and astrocytoma patients, in contrast to our findings, where WHO grade was only relevant in oligodendroglioma patients (Suppl. Fig. 2b and Fig. 2d and e). Taken together, comparing our results with the cohort of TCGA, yielded similar results, increasing the validity of our findings.
Discussion
This study analyzes a molecularly characterized cohort of supratentorial IDHmut glioma patients with special emphasis on patients above the age of 60 (elderly) which comprised only 10% of the cohort. Younger (< 60 years) and elderly patients were comparable in most patient-, tumor- and treatment-related factors except for preoperative KPS (lower in the elderly), SVZ involvement (more frequent in the elderly) and tumor subtype (oligodendroglioma more frequent in the elderly). Midline crossing of the tumor, pre- and post-operative tumor volumes and the choice of surgical strategy influenced PFS but were independent of patient age. Importantly, treatment intensity in terms of surgical strategy, residual tumor volumes and adjuvant treatment were equally high and postoperative morbidity was equally low in both age groups, illustrating that elderly LGG patients can be treated as aggressively as younger patients as PFS did not differ.
The predicted incidence of astrocytoma and oligodendroglioma in the USA is 0.51 and 0.25 per 100,000 inhabitants per year; they account for only 6.4% of all adult primary central nervous tumors [19]. In this study cohort, only 10% of LGG patients were above the age of 60 years. Considering that about 500 primary brain tumor operations are being performed in our department each year, only three elderly LGG patients are being diagnosed per year. This circumstance makes it virtually impossible to recruit enough patients to power randomized-controlled trials (RCT) and explains the sparse literature on this rare patient population. In lack of RCTs, analysis of real-world data is a feasible alternative to understand the treatment outcomes in a broader, representative patient population as presented in this study [20].
This work focuses exclusively on IDHmut astrocytoma and oligodendroglioma, the “lower-grade glioma” patients. This is of importance since IDHwt astrocytoma, independent of WHO grade, has been shown to confer an unfavorable prognosis and therefore is now being classified as GBM IDHwt [2, 21, 22]. Since GBM typically presents at a median age of 65, it is likely that previous studies on elderly LGG patients unintentionally included GBM patients which distorts reports on patient outcome [19]. A recent analysis by Morshed and colleagues reported on 26 WHO grade 2 glioma patients aged 60 or older. However, 26.9% harbored astrocytoma IDHwt tumors, resulting in an overall PFS of 23.5 months [10]. In contrast, we report a PFS twice as long (46 months) which was also comparable to that of younger IDHmut patients.
Comparing our results to the TCGA cohort, we found coinciding distributions of tumor subtypes and WHO grades in the younger and elderly undermining the validity of our findings. Interestingly, in contrast to the TCGA cohort, OS did not differ between astrocytomas WHO grade 2 and 3 in our study. According to a recent review by Von Deimling et al. analyzing studies conducted pre and post 2016, this circumstance can be ascribed to changes in classification following the 2016 CNS WHO update, while the criteria for grading remained largely untouched leading to a striking reduction of prognostic significance of diffuse astrocytic glioma grading which was also described by Olar et al. [23, 24].
In this analysis, elderly and younger patients differed only in few aspects. Interestingly, SVZ involvement was more frequent in elderly patients, a finding which hasn’t been described before. In GBM IDHwt, SVZ involvement has been linked to inferior survival and distinct growth and recurrence patterns [25]. In LGG patients, however, reports on SVZ involvement are sparse and warrant further investigations. While both age groups did not differ with respect to WHO grade, oligodendroglioma was more frequent in the elderly. This has been reported before and may help to explain the favorable outcome in this age population [26].
Not surprisingly, the median KPS of elderly patients was 8 points below the younger. Despite this small but measurable difference in functional status, treatment was not restrained in the elderly, and KPS per se did not affect PFS. Unlike in glioblastoma IDHwt where treatment recommendations differ considerably in younger and elderly (> 65 years) patients, there are no evidence-based treatment recommendations in elderly LGG patients [27,28,29]. Since the initial report by Pignatti et al., a patients’ age above 40 has been considered “high risk” for poor outcome and has therefore been incorporated as a stratification factor in large-scale RCTs and evidence-based treatment recommendations [1, 5,6,7]. This may in part explain why adjuvant treatment was frequently administered in our younger patient cohort as well (61% as compared to 76% in the elderly).
In our cohort, surgical strategy was independent of age. Most patients underwent tumor resection (81% of the elderly vs. 91% of the younger patients) with comparable residual FLAIR and CE tumor volumes and low surgical morbidity in both age groups, demonstrating that maximized resection can be safely pursued in the elderly as well. This contrasts with a previous report by Youland et al. reporting on their 50-year experience on patients over 55 years harboring a WHO grade 2 glioma where resection was only performed in 36% of cases [11]. This reflects different treatment strategies which, of course, may have changed over time. Also, recent studies report a more favorable “resectability” of IDH mutant tumors compared to historical IDHwt WHO “grade 2” tumors [30, 31]. In univariate survival analysis, tumor resection as well as smaller residual tumor volumes were identified as prognostic factors for prolonged PFS, both in the overall cohort and in subgroup analysis, undermining the prognostic relevance of tumor resection in LGG patients [32, 33].
As with all retrospective analyses, there are several limitations. Treatment decisions were uncontrolled and may have biased our results. Also, the imbalance between the much larger younger and the small elderly cohort (186 vs. 21 patients) may affect statistical analysis and mask effects otherwise detected. Nevertheless, considering the very low incidence of elderly LGG patients, analysis of real-world data appears feasible to unravel patient characteristics and treatment outcomes.
Taken together, PFS and cancer-related survival were comparable in elderly and younger patients, owing to the exclusion of IDHwt tumors, but also to a high treatment intensity in both groups. This demonstrates, that elderly LGG patients have tumor properties and treatment outcomes like younger patients and should encourage clinicians not to restrain from intensified treatment.
Conclusion
This study presents a molecularly well-characterized, contemporary cohort of elderly lower-grade glioma patients. Younger and elderly patients conferred comparable surgical outcomes and PFS. Intensified treatment including maximal safe resection should therefore be advocated in elderly lower-grade glioma patients whenever feasible.
References
Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, Bendszus M, Balana C, Chinot O, Dirven L et al (2021) EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 18:170–186. https://doi.org/10.1038/s41571-020-00447-z
Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, Hawkins C, Ng HK, Pfister SM, Reifenberger G et al (2021) The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-Oncology 23:1231–1251. https://doi.org/10.1093/neuonc/noab106
Hartmann C, Hentschel B, Wick W, Capper D, Felsberg J, Simon M, Westphal M, Schackert G, Meyermann R, Pietsch T et al (2010) Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol 120:707–718. https://doi.org/10.1007/s00401-010-0781-z
Cancer Genome Atlas Research Network, Brat DJ, Verhaak RGW, Aldape KD, Yung WKA, Salama SR, Cooper LAD, Rheinbay E, Miller CR, Vitucci M et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498. https://doi.org/10.1056/NEJMoa1402121
Pignatti F, van den Bent M, Curran D, Debruyne C, Sylvester R, Therasse P, Afra D, Cornu P, Bolla M, Vecht C et al (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084. https://doi.org/10.1200/JCO.2002.08.121
Shaw EG, Wang M, Coons SW, Brachman DG, Buckner JC, Stelzer KJ, Barger GR, Brown PD, Gilbert MR, Mehta MP (2012) Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: initial results of RTOG 9802. J Clin Oncol 30:3065–3070. https://doi.org/10.1200/JCO.2011.35.8598
Baumert BG, Hegi ME, van den Bent MJ, von Deimling A, Gorlia T, Hoang-Xuan K, Brandes AA, Kantor G, Taphoorn MJB, Hassel MB et al (2016) Temozolomide chemotherapy versus radiotherapy in high-risk low-grade glioma (EORTC 22033-26033): a randomised, open-label, phase 3 intergroup study. Lancet Oncol 17:1521–1532. https://doi.org/10.1016/S1470-2045(16)30313-8
Hartmann C, Meyer J, Balss J, Capper D, Mueller W, Christians A, Felsberg J, Wolter M, Mawrin C, Wick W et al (2009) Type and frequency of IDH1 and IDH2 mutations are related to astrocytic and oligodendroglial differentiation and age: a study of 1,010 diffuse gliomas. Acta Neuropathol 118:469–474. https://doi.org/10.1007/s00401-009-0561-9
Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD, Kleihues P, Ellison DW (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820. https://doi.org/10.1007/s00401-016-1545-1
Morshed RA, Young JS, Hervey-Jumper SL, Berger MS (2019) The management of low-grade gliomas in adults. J Neurosurg Sci 63:450–457. https://doi.org/10.23736/S0390-5616.19.04701-5
Youland RS, Schomas DA, Brown PD, Parney IF, Laack NNI (2017) Patterns of care and treatment outcomes in older adults with low grade glioma: a 50-year experience. J Neurooncol 133:339–346. https://doi.org/10.1007/s11060-017-2439-3
Pouratian N, Mut M, Jagannathan J, Lopes MB, Shaffrey ME, Schiff D (2008) Low-grade gliomas in older patients: a retrospective analysis of prognostic factors. J Neurooncol 90:341–350. https://doi.org/10.1007/s11060-008-9669-3
Kaloshi G, Psimaras D, Mokhtari K, Dehais C, Houillier C, Marie Y, Laigle-Donadey F, Taillibert S, Guillevin R, Martin-Duverneuil N et al (2009) Supratentorial low-grade gliomas in older patients. Neurology 73:2093–2098. https://doi.org/10.1212/WNL.0b013e3181c6781e
Schomas DA, Laack NN, Brown PD (2009) Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer 115:3969–3978. https://doi.org/10.1002/cncr.24444
Kumthekar P, Patel V, Bridge C, Rademaker A, Helenowski I, Mrugala MM, Rockhill JK, Grimm S, Swanson KR, Raizer J (2017) Prognosis of older patients with low-grade glioma: a retrospective study. Integr Cancer Sci Ther. https://doi.org/10.15761/icst.1000255
Capper D, Weissert S, Balss J, Habel A, Meyer J, Jäger D, Ackermann U, Tessmer C, Korshunov A, Zentgraf H et al (2010) Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors. Brain Pathol 20:245–254. https://doi.org/10.1111/j.1750-3639.2009.00352.x
Capper D, Stichel D, Sahm F, Jones DTW, Schrimpf D, Sill M, Schmid S, Hovestadt V, Reuss DE, Koelsche C et al (2018) Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol 136:181–210. https://doi.org/10.1007/s00401-018-1879-y
Brennan CW, Verhaak RGW, McKenna A, Campos B, Noushmehr H, Salama SR, Zheng S, Chakravarty D, Sanborn JZ, Berman SH et al (2013) The somatic genomic landscape of glioblastoma. Cell 155:462–477. https://doi.org/10.1016/j.cell.2013.09.034
Ostrom QT, Cioffi G, Waite K, Kruchko C, Barnholtz-Sloan JS (2021) CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2014–2018. Neuro-Oncology 23:iii1–iii105. https://doi.org/10.1093/neuonc/noab200
Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS (2018) Interpretation and impact of real-world clinical data for the practicing clinician. Adv Ther 35:1763–1774. https://doi.org/10.1007/s12325-018-0805-y
Reuss DE, Kratz A, Sahm F, Capper D, Schrimpf D, Koelsche C, Hovestadt V, Bewerunge-Hudler M, Jones DTW, Schittenhelm J et al (2015) Adult IDH wild type astrocytomas biologically and clinically resolve into other tumor entities. Acta Neuropathol 130:407–417. https://doi.org/10.1007/s00401-015-1454-8
Hasselblatt M, Jaber M, Reuss D, Grauer O, Bibo A, Terwey S, Schick U, Ebel H, Niederstadt T, Stummer W et al (2018) Diffuse astrocytoma, IDH-wildtype: a dissolving diagnosis. J Neuropathol Exp Neurol 77:422–425. https://doi.org/10.1093/jnen/nly012
von Deimling A, Ono T, Shirahata M, Louis DN (2018) Grading of diffuse astrocytic gliomas: a review of studies before and after the advent of IDH testing. Semin Neurol 38:19–23. https://doi.org/10.1055/s-0038-1636430
Olar A, Wani KM, Alfaro-Munoz KD, Heathcock LE, van Thuijl HF, Gilbert MR, Armstrong TS, Sulman EP, Cahill DP, Vera-Bolanos E et al (2015) IDH mutation status and role of WHO grade and mitotic index in overall survival in grade II–III diffuse gliomas. Acta Neuropathol 129:585–596. https://doi.org/10.1007/s00401-015-1398-z
Jungk C, Warta R, Mock A, Friauf S, Hug B, Capper D, Abdollahi A, Debus J, Bendszus M, von Deimling A et al (2019) Location-dependent patient outcome and recurrence patterns in IDH1-wildtype glioblastoma. Cancers (Basel). https://doi.org/10.3390/cancers11010122
Morshed RA, Han SJ, Hervey-Jumper SL, Pekmezci M, Troncon I, Chang SM, Butowski NA, Berger MS (2019) Molecular features and clinical outcomes in surgically treated low-grade diffuse gliomas in patients over the age of 60. J Neurooncol 141:383–391. https://doi.org/10.1007/s11060-018-03044-4
Wick W, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, Nikkhah G, Papsdorf K, Steinbach JP, Sabel M et al (2012) Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol 13:707–715. https://doi.org/10.1016/S1470-2045(12)70164-X
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. https://doi.org/10.1056/NEJMoa043330
Pellerino A, Bruno F, Internò V, Rudà R, Soffietti R (2020) Current clinical management of elderly patients with glioma. Expert Rev Anticancer Ther 20:1037–1048. https://doi.org/10.1080/14737140.2020.1828867
Beiko J, Suki D, Hess KR, Fox BD, Cheung V, Cabral M, Shonka N, Gilbert MR, Sawaya R, Prabhu SS et al (2014) IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-Oncology 16:81–91. https://doi.org/10.1093/neuonc/not159
Jones PS, Carroll KT, Koch M, DiCesare JAT, Reitz K, Frosch M, Barker FG, Cahill DP, Curry WT (2019) Isocitrate dehydrogenase mutations in low-grade gliomas correlate with prolonged overall survival in older patients. Neurosurgery 84:519–528. https://doi.org/10.1093/neuros/nyy149
Jakola AS, Myrmel KS, Kloster R, Torp SH, Lindal S, Unsgård G, Solheim O (2012) Comparison of a strategy favoring early surgical resection vs a strategy favoring watchful waiting in low-grade gliomas. JAMA 308:1881–1888. https://doi.org/10.1001/jama.2012.12807
Scherer M, Ahmeti H, Roder C, Gessler F, Jungk C, Pala A, Mayer B, Senft C, Tatagiba M, Synowitz M et al (2019) Surgery for diffuse WHO Grade II gliomas: volumetric analysis of a multicenter retrospective cohort from the German Study Group for Intraoperative Magnetic Resonance Imaging. Neurosurgery. https://doi.org/10.1093/neuros/nyz397
Funding
Open Access funding enabled and organized by Projekt DEAL. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
The study was designed by CJ and PDT. Material preparation, data collection and analysis were performed by CJ, MG and PDT. Resources were provided by AU, CH-M, DR, AD, AW, JD and LK. The first draft of the manuscript was written by PDT and CJ and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of the Medical Faculty of the University of Heidelberg (reference S-005/2003, as of 31.01.2003).
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dao Trong, P., Gluszak, M., Reuss, D. et al. Isocitrate-dehydrogenase-mutant lower grade glioma in elderly patients: treatment and outcome in a molecularly characterized contemporary cohort. J Neurooncol 161, 605–615 (2023). https://doi.org/10.1007/s11060-022-04230-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-022-04230-1