Abstract
Purpose
WHO grade II gliomas are uncommon in patients over the age of 60, and there is a lack in consensus regarding their management. We present molecular tumor characteristics as well as clinical outcomes in patients over the age of 60 undergoing surgical resection of a WHO grade II glioma.
Methods
After receiving IRB approval, patients were identified through the UCSF Brain Tumor Center. Pathologic diagnosis was completed using WHO 2016 grading criteria.
Results
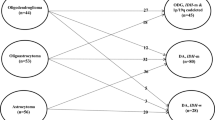
Twenty-six patients with a mean age of 66 years met inclusion criteria with a median follow-up of 5.2 years. Diagnoses included diffuse astrocytoma IDH-mutant (19.2%), diffuse astrocytoma IDH-wildtype (26.9%), Oligodendroglioma IDH-mutant and 1p/19q-codeleted (50%), and a rare case of mixed oligoastrocytoma (3.9%). 66% of astrocytoma IDH-wildtype tumors possessed TERT mutation. Median extent of resection was 75.4%. Progression-free (PFS) and overall survival (OS) were 23.5 and 62.6 months, respectively. Shorter PFS was associated with the astrocytoma IDH-wildtype subtype despite similar extent of resection and adjuvant treatment rates compared to the other subtypes. OS did not differ between subtypes. Malignant transformation and death were associated with larger preoperative and residual tumor volume.
Conclusions
Older patients with diffuse gliomas may safely undergo aggressive treatment with surgical resection and adjuvant therapy. Elderly patients with low grade gliomas have worse clinical outcomes compared to their younger counterparts. This may be due to an increased frequency of diffuse astrocytoma IDH-wildtype tumors in this age group.

Similar content being viewed by others
References
Pekmezci M, Rice T, Molinaro AM et al (2017) Adult infiltrating gliomas with WHO 2016 integrated diagnosis: additional prognostic roles of ATRX and TERT. Acta Neuropathol. https://doi.org/10.1007/s00401-017-1690-1
Eckel-Passow JE, Lachance DH, Molinaro AM et al (2015) Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 372:2499–2508. https://doi.org/10.1056/NEJMoa1407279
Rasmussen BK, Hansen S, Laursen RJ et al (2017) Epidemiology of glioma: clinical characteristics, symptoms, and predictors of glioma patients grade I–IV in the the Danish Neuro-Oncology Registry. J Neurooncol. https://doi.org/10.1007/s11060-017-2607-5
Network CGAR, Brat DJ, Verhaak RGW et al (2015) Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N Engl J Med 372:2481–2498. https://doi.org/10.1056/NEJMoa1402121
Lote K, Egeland T, Hager B et al (1997) Survival, prognostic factors, and therapeutic efficacy in low-grade glioma: a retrospective study in 379 patients. J Clin Oncol 15:3129–3140. https://doi.org/10.1200/JCO.1997.15.9.3129
Okamoto Y, Di Patre PL, Burkhard C et al (2004) Population-based study on incidence, survival rates, and genetic alterations of low-grade diffuse astrocytomas and oligodendrogliomas. Acta Neuropathol 108:49–56. https://doi.org/10.1007/s00401-004-0861-z
Chang EF, Smith JS, Chang SM et al (2008) Preoperative prognostic classification system for hemispheric low-grade gliomas in adults. J Neurosurg 109:817–824. https://doi.org/10.3171/JNS/2008/109/11/0817
Davis FG, Freels S, Grutsch J et al (1998) Survival rates in patients with primary malignant brain tumors stratified by patient age and tumor histological type: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, 1973-1991. J Neurosurg 88:1–10. https://doi.org/10.3171/jns.1998.88.1.0001
Pignatti F, Van den Bent M, Curran D et al (2002) Prognostic factors for survival in adult patients with cerebral low-grade glioma. J Clin Oncol 20:2076–2084. https://doi.org/10.1200/JCO.2002.08.121
Shaw E, Arusell R, Scheithauer B et al (2002) Prospective randomized trial of low-versus high-dose radiation therapy in adults with supratentorial low-grade glioma: initial report of a North Central Cancer Treatment Group/Radiation Therapy Oncology Group/Eastern Cooperative Oncology Group Study. J Clin Oncol 20:2267–2276. https://doi.org/10.1200/JCO.2002.09.126
Kaloshi G, Psimaras D, Mokhtari K et al (2009) Supratentorial low-grade gliomas in older patients. Neurology 73:2093–2098. https://doi.org/10.1212/WNL.0b013e3181c6781e
Schomas DA, Laack NN, Brown PD (2009) Low-grade gliomas in older patients: long-term follow-up from Mayo Clinic. Cancer 115:3969–3978. https://doi.org/10.1002/cncr.24444
Capelle L, Fontaine D, Mandonnet E et al (2013) Spontaneous and therapeutic prognostic factors in adult hemispheric World Health Organization Grade II gliomas: a series of 1097 cases. J Neurosurg 118:1157–1168. https://doi.org/10.3171/2013.1.JNS121
Buckner JC, Shaw EG, Pugh SL et al (2016) Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med 374:1344–1355. https://doi.org/10.1056/NEJMoa1500925
Berger MS, Deliganis AV, Dobbins J, Keles GE (1994) The effect of extent of resection on recurrence in patients with low grade cerebral hemisphere gliomas. Cancer 74:1784–1791. https://doi.org/10.1002/1097-0142(19940915)74:6%3C1784::AID-CNCR2820740622%3E3.0.CO;2-D
Keles GE, Lamborn KR, Berger MS (2001) Low-grade hemispheric gliomas in adults: a critical review of extent of resection as a factor influencing outcome. J Neurosurg 95:735–745. https://doi.org/10.3171/jns.2001.95.5.0735
Iwamoto FM, Reiner AS, Nayak L et al (2009) Prognosis and patterns of care in elderly patients with glioma. Cancer 115:5534–5540. https://doi.org/10.1002/cncr.24612
International Agency for Research on Cancer (2016) WHO Classification of Tumours of the Central Nervous System, 4th edn. World Health Organization, Geneva
National Center for Health Statistics (2017) Health, United States, 2016. Hyattsville
Pouratian N, Mut M, Jagannathan J et al (2008) Low-grade gliomas in older patients: a retrospective analysis of prognostic factors. J Neurooncol 90:341–350. https://doi.org/10.1007/s11060-008-9669-3
Chi A, Batchelor T (2007) Low-grade gliomas in older patients: a malignant tumor? Neuro Oncol 9:545
Smith JS, Chang EF, Lamborn KR et al (2008) Role of extent of resection in the long-term outcome of low-grade hemispheric gliomas. J Clin Oncol 26:1338–1345. https://doi.org/10.1200/JCO.2007.13.9337
Murphy ES, Leyrer CM, Parsons M et al (2018) Risk factors for malignant transformation of low-grade glioma. Int J Radiat Oncol Biol Phys 100:965–971. https://doi.org/10.1016/j.ijrobp.2017.12.258
Brat DJ, Aldape K, Colman H et al (2018) cIMPACT-NOW update 3: recommended diagnostic criteria for “Diffuse astrocytic glioma, IDH-wildtype, with molecular features of glioblastoma, WHO grade IV”. Acta Neuropathol. https://doi.org/10.1007/s00401-018-1913-0
Hervey-Jumper SL, Li J, Osorio JA et al (2016) Surgical assessment of the insula. Part 2: validation of the Berger-Sanai zone classification system for predicting extent of glioma resection. J Neurosurg. https://doi.org/10.3171/2015.4.JNS1521
Sanai N, Polley M-Y, Berger MS (2010) Insular glioma resection: assessment of patient morbidity, survival, and tumor progression. J Neurosurg. https://doi.org/10.3171/2009.6.JNS0952
Acknowledgements
We would like to thank Jing Li for completing the search of the institutional database for patients qualifying for this study.
Author information
Authors and Affiliations
Contributions
All authors have contributed significantly to the experimental design, analyses, interpretation of the data and writing of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Morshed, R.A., Han, S.J., Hervey-Jumper, S.L. et al. Molecular features and clinical outcomes in surgically treated low-grade diffuse gliomas in patients over the age of 60. J Neurooncol 141, 383–391 (2019). https://doi.org/10.1007/s11060-018-03044-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-018-03044-4