Abstract
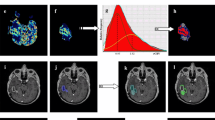
We analyzed whether the combined visualization of decreased apparent diffusion coefficient (ADC) values and increased cerebral blood volume (CBV) in perfusion imaging can identify prognosis-related growth patterns in patients with newly diagnosed glioblastoma. Sixty-five consecutive patients were examined with diffusion and dynamic susceptibility-weighted contrast-enhanced perfusion weighted MRI. ADC and CBV maps were co-registered on the T1-w image and a region of interest (ROI) was manually delineated encompassing the enhancing lesion. Within this ROI pixels with ADC values <the 30th percentile (ADCmin), pixels with CBV values >the 70th percentile (CBVmax) and the intersection of pixels with ADCmin and CBVmax were automatically calculated and visualized. Initially, all tumors with a mean intersection greater than the upper quartile of the normally distributed mean intersection of all patients were subsumed to the first growth pattern termed big intersection (BI). Subsequently, the remaining tumors’ growth patterns were categorized depending on the qualitative representation of ADCmin, CBVmax and their intersection. Log-rank test exposed a significantly longer overall survival of BI (n = 16) compared to non-BI group (n = 49) (p = 0.0057). Thirty-one, four and 14 patients of the non-BI group were classified as predominant ADC-, CBV- and mixed growth group, respectively. In a multivariate Cox regression model, the BI-, CBV- and mixed groups had significantly lower adjusted hazard ratios (p-value, αBonferroni < 0.006) when compared to the reference group ADC: 0.29 (0.0027), 0.11 (0.038) and 0.33 (0.0059). Our study provides evidence that the combination of diffusion and perfusion imaging allows visualization of different glioblastoma growth patterns that are associated with prognosis. A possible biological hypothesis for this finding could be the interpretation of the ADCmin fraction as the invasion-front of tumor cells while the CBVmax fraction might represent the vascular rich tumor border that is “trailing behind” the invasion-front in the ADC group.



Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
Stupp R, Hegi ME, Mason WP et al (2009) Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 10:459–466
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Field K, Rosenthal M, Yilmaz M, Tacey M, Drummond KJ (2014) Comparison between poor and long-term survivors with glioblastoma: review of an Australian dataset. Asia Pac J Clin Oncol 10:153–161
Hartmann C, Hentschel B, Simon M et al (2013) Long-term survival in primary glioblastoma with versus without isocitrate dehydrogenase mutations. Clin Cancer Res 19:5146–5157
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds) (2007) WHO classification of tumours of the central nervous system, 4th edn. IARC, Lyon, pp 33–52
Chawalparit O, Sangruchi T, Witthiwej T et al (2013) Diagnostic performance of advanced MRI in differentiating high-grade from low-grade gliomas in a setting of routine service. J Med Assoc Thai 96:1365–1373
Wang S, Zhou J (2012) Diffusion tensor magnetic resonance imaging of rat glioma models: a correlation study of MR imaging and histology. J Comput Assist Tomogr 36:739–744
Weber M, Henze M, Tuttenberg J et al (2010) Biopsy targeting gliomas: do functional imaging techniques identify similar target areas? Investig Radiol 45:755–768
Weber M, Zoubaa S, Schlieter M et al (2006) Diagnostic performance of spectroscopic and perfusion MRI for distinction of brain tumors. Neurology 66:1899–1906
Murakami R, Hirai T, Sugahara T et al (2009) Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot method. Radiology 251:838–845
Sottoriva A, Spiteri I, Piccirillo SGM et al (2013) Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc Natl Acad Sci USA 110:4009–4014
Jacobs AH, Kracht LW, Gossmann A et al (2005) Imaging in neurooncology. NeuroRx 2:333–347
Burger PC, Vogel FS, Green SB, Strike TA (1985) Glioblastoma multiforme and anaplastic astrocytoma. Pathologic criteria and prognostic implications. Cancer 56:1106–1111
Romano A, Calabria LF, Tavanti F et al (2013) Apparent diffusion coefficient obtained by magnetic resonance imaging as a prognostic marker in glioblastomas: correlation with MGMT promoter methylation status. Eur Radiol 23:513–520
Aronen HJ, Gazit IE, Louis DN et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Radbruch A, Bendszus M, Wick W, Heiland S (2010) Comment to: parametric response map as an imaging biomarker to distinguish progression from pseudoprogression in high-grade glioma: pitfalls in perfusion MRI in brain tumors : Tsien C, Galban CJ, Chenevert TL, Johnson TD, Hamstra DA, Sundgren PC, Junck L. Clin Neuroradiol 20:183–184
Maes F, Collignon A, Vandermeulen D, Marchal G, Suetens P (1997) Multimodality image registration by maximization of mutual information. IEEE Trans Med Imaging 16:187–198
Meyer CR, Boes JL, Kim B et al (1997) Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Med Image Anal 1:195–206
Pluim JPW, Maintz JBA, Viergever MA (2003) Mutual-information-based registration of medical images: a survey. IEEE Trans Med Imaging 22:986–1004
Marko NF, Weil RJ, Schroeder JL, Lang FF, Suki D, Sawaya RE (2014) Extent of resection of glioblastoma revisited: personalized survival modeling facilitates more accurate survival prediction and supports a maximum-safe-resection approach to surgery. J Clin Oncol 32:774–782
Kaur B, Khwaja FW, Severson EA, Matheny SL, Brat DJ, Van Meir EG (2005) Hypoxia and the hypoxia-inducible-factor pathway in glioma growth and angiogenesis. Neuro Oncol 7:134–153
Fujiwara S, Nakagawa KOU, Harada H et al (2007) Silencing hypoxia-inducible factor-1 · inhibits cell migration and invasion under hypoxic environment in malignant gliomas. Int J Oncol 30:793–802
Zagzag D, Lukyanov Y, Lan L et al (2006) Hypoxia-inducible factor 1 and VEGF upregulate CXCR4 in glioblastoma: implications for angiogenesis and glioma cell invasion. Lab Investig 86:1221–1232
Giese A, Loo MA, Tran N, Haskett D, Coons SW, Berens ME (1996) Dichotomy of astrocytoma migration and proliferation. Int J Cancer 67:275–282
St Croix B, Kerbel RS (1997) Cell adhesion and drug resistance in cancer. Curr Opin Oncol 9:549–556
McAuliffe MJ, Lalonde FM, McGarry D, Gandler W, Csaky K, Trus BL (2001) Medical image processing, analysis & visualization in clinical research. Computer-Based Medical Systems, pp 381–386
Rosset A, Spadola L, Ratib O (2004) OsiriX: an open-source software for navigating in multidimensional DICOM images. J Digit Imaging 17:205–216
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Authors’ contributions
KD: Study concept, Study design, Literature research, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation. BW: Clinical studies, Data acquisition, Data analysis, Literature Research, Manuscript editing. MG: Data analysis, Manuscript editing. CR: Literature Research. ROF: Data analysis, Manuscript editing. PB: Manuscript editing. PK: Manuscript editing. SH: Manuscript editing. HPS: Manuscript editing. WW: Manuscript editing. MB: Manuscript editing. AR: Study concept, Study design, Literature research, Clinical studies, Data acquisition, Data analysis, Statistical analysis, Manuscript preparation, Guarantor of the integrity of the entire study, Study coordination.
Funding
This study was supported by Guerbet (Paris) and by “Intramurales Förderprogramm”, German Cancer Research Center, DKFZ, Heidelberg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors indicate no potential conflicts of interest.
Additional information
Katerina Deike and Benedikt Wiestler contributed equally to the manuscript.
Statistical analysis was conducted by Katerina Deike and Alexander Radbruch.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Deike, K., Wiestler, B., Graf, M. et al. Prognostic value of combined visualization of MR diffusion and perfusion maps in glioblastoma. J Neurooncol 126, 463–472 (2016). https://doi.org/10.1007/s11060-015-1982-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1982-z