Abstract
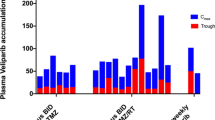
Iniparib is a prodrug that converts to highly reactive cytotoxic metabolites intracellularly with activity in preclinical glioma models. We investigated the maximum tolerated dose (MTD) of iniparib with monthly (m) and continuous (c) temozolomide (TMZ) dosing schedules in patients with malignant gliomas (MG). Adults with newly diagnosed MG who had successfully completed ≥80 % of radiation (RT) and TMZ without toxicity received mTMZ dosing (150–200 mg/m2 days 1–5/28 days) or cTMZ dosing (75 mg/m2/days × 6 weeks) in conjunction with iniparib (i.v. 2 days/week) in the adjuvant setting. Iniparib was dose escalated using a modified continual reassessment method (mCRM). 43 patients (32 male; 34 GBM, 8 AA, 1 gliosarcoma; median age 54 years; median KPS 90) were enrolled across 4 dose levels. In the mTMZ group, 2/4 patients had dose limiting toxicities (DLT) at 19 mg/kg/week (rash/hypersensitivity). At 17.2 mg/kg/week, 1/9 patients had a DLT (grade 3 fatigue). Additional grade 3 toxicities were neutropenia, lymphopenia, and nausea. In the cTMZ group, one DLT (thromboembolic event) occurred at 10.2 mg/kg/week. Dose escalation stopped at 16 mg/kg/week based on mCRM. The mean maximum plasma concentration of iniparib increased with dose. Concentration of the two major circulating metabolites, 4-iodo-3-aminobenzamide and 4-iodo-3-aminobenzoic acid, was ≤5 % of the corresponding iniparib concentration. Iniparib is well tolerated, at doses higher than previously investigated, in combination with TMZ after completion of RT + TMZ in patients with MG. Recommended phase 2 dosing of iniparib based on mCRM is 17.2 mg/kg/week with mTMZ and 16 mg/kg/week with cTMZ. An efficacy study of TMZ/RT + iniparib followed by TMZ + iniparib in newly diagnosed GBM using these doses has completed enrollment. Survival assessment is ongoing.
Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. doi:10.1056/NEJMoa043330
Hegi ME, Liu L, Herman JG, Stupp R, Wick W, Weller M, Mehta MP, Gilbert MR (2008) Correlation of O6-methylguanine methyltransferase (MGMT) promoter methylation with clinical outcomes in glioblastoma and clinical strategies to modulate MGMT activity. J Clin Oncol 26:4189–4199. doi:10.1200/JCO.2007.11.5964
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. doi:10.1056/NEJMoa043331
Ossovskaya V, Lim C, Schools G, Kalurupalle S, Roninson I, Broude E (2011) The chemosensitizing properties of iniparib in combination with DNA-damaging agents in the MDA-MB -468(–) triple-negative breast cancer (TNBC) cell line. AACR annual meeting : abstract LB-401
BiPar sciences personal communication (2011) BSI-201 Investigator’s Brochure
Patel AG, De Lorenzo SB, Flatten KS, Poirier GG, Kaufmann SH (2012) Failure of iniparib to inhibit poly(ADP-Ribose) polymerase in vitro. Clin Cancer Res 18:1655–1662. doi:10.1158/1078-0432.CCR-11-2890
Mahany JJ, Lewis N, Heath EI, LoRusso PM, Mita MM, Rodon J, Tolcher AW, Sherman BM, Bradley CR, Papadopoulos KP (2008) A phase IB study evaluating BSI-201 in combination with chemotherapy in subjects with advanced solid tumors. J Clin Oncol 26:3579
Liu X, Shi Y, Maag DX, Palma JP, Patterson MJ, Ellis PA, Surber BW, Ready DB, Soni NB, Ladror US et al (2012) Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res 18:510–523. doi:10.1158/1078-0432.CCR-11-1973
Mendeleyev J, Kirsten E, Hakam A, Buki KG, Kun E (1995) Potential chemotherapeutic activity of 4-iodo-3-nitrobenzamide. Metabolic reduction to the 3-nitroso derivative and induction of cell death in tumor cells in culture. Biochem Pharmacol 50:705–714
Licht S, Cao H, Li Z, Zhang J, Liu F, Brittain S, Shen J, Zhang B, Hopke J, Newcombe R, et al (2011) Mechanism of action of iniparib: stimulation of reactive oxygen species (ROS) production in an iniparib-sensitive breast cancer cell line. American Association for Cancer Research, San Francisco, nrA226
Castro M, Li L, Stallings TE (2010) Pharmacokinetics of BSI-201, a poly (ADP-ribose) polymerase-1 (PARP1) inhibitor, in cerebrospinal fluid (CSF) of a patient with breast cancer with carcinomatous meningitis. J Clin Oncol, No. 15_suppl (May 20 Supplement): e13559
O’Shaughnessy J, Osborne C, Pippen JE, Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM, Bradley C (2011) Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N Engl J Med 364:205–214. doi:10.1056/NEJMoa1011418
Novello S, Besse B, Felip E, Barlesi F, Mazieres J, Zalcman G, von Pawel J, Reck M, Cappuzzo F, Ferry D et al (2014) A phase II randomized study evaluating the addition of iniparib to gemcitabine plus cisplatin as first-line therapy for metastatic non-small-cell lung cancer. Ann Oncol 25:2156–2162. doi:10.1093/annonc/mdu384
Aghajanian C, Sill MW, Secord AA, Powell MA, Steinhoff M (2012) Iniparib plus paclitaxel and carboplatin as initial treatment of advanced or recurrent uterine carcinosarcoma: a gynecologic oncology group study. Gynecol Oncol 126:424–427. doi:10.1016/j.ygyno.2012.05.024
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Grossman SA, Carson KA, Phuphanich S, Batchelor T, Peereboom D, Nabors LB, Lesser G, Hausheer F, Supko JG (2008) New approaches to brain tumor therapy CNS consortium phase I and pharmacokinetic study of karenitecin in patients with recurrent malignant gliomas. Neuro Oncol 10:608–616. doi:10.1215/15228517-2008-030
Piantadosi S, Fisher JD, Grossman S (1998) Practical implementation of a modified continual reassessment method for dose-finding trials. Cancer Chemother Pharmacol 41:429–436
Piantadosi S (2005) Clinical trials: a methodologic perspective, 2nd edn. Wiley, New York
Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Stat Assoc 53:457–481
Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38(1):29–41
Gilbert MR, Wang M, Aldape KD, Stupp R, Hegi ME, Jaeckle KA, Armstrong TS, Wefel JS, Won M, Blumenthal DT et al (2013) Dose-dense temozolomide for newly diagnosed glioblastoma: a randomized phase III clinical trial. J Clin Oncol 31:4085–4091. doi:10.1200/JCO.2013.49.6968
Brada M, Hoang-Xuan K, Rampling R, Dietrich PY, Dirix LY, Macdonald D, Heimans JJ, Zonnenberg BA, Bravo-Marques JM, Henriksson R et al (2001) Multicenter phase II trial of temozolomide in patients with glioblastoma multiforme at first relapse. Ann Oncol 12:259–266
Yung WK, Albright RE, Olson J, Fredericks R, Fink K, Prados MD, Brada M, Spence A, Hohl RJ, Shapiro W et al (2000) A phase II study of temozolomide vs. procarbazine in patients with glioblastoma multiforme at first relapse. Br J Cancer 83:588–593. doi:10.1054/bjoc.2000.1316
Acknowledgments
The authors thank Susan Passwaters for assistance with administrative management of the manuscript; Joy Fisher for assistance with data collection oversight on behalf of the Adult Brain Tumor Consortium and the team at Cancer Therapy Evaluation Program for their oversight and collaboration through the Adult Brain Tumor Consortium.
Funding
National Institutes of Health (U01 CA-62475 to S.G., U01 CA-137443 to S.G.); Sanofi Pharmaceuticals, Inc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Authors declare they have no conflict of interest.
Additional information
On behalf of the Adult Brain Tumor Consortium.
Rights and permissions
About this article
Cite this article
Blakeley, J.O., Grossman, S.A., Mikkelsen, T. et al. Phase I study of iniparib concurrent with monthly or continuous temozolomide dosing schedules in patients with newly diagnosed malignant gliomas. J Neurooncol 125, 123–131 (2015). https://doi.org/10.1007/s11060-015-1876-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-015-1876-0