Abstract
Purpose
A multi-site Phase I trial was conducted to determine the safety, maximum tolerated dose, and pharmacokinetics (PK) of Veliparib, a Poly (ADP-ribose) polymerase [PARP] enzyme inhibitor, when administered with temozolomide (TMZ) alone and then with temozolomide and radiation (RT) in patients with newly diagnosed glioblastoma.
Methods
Given the potential for myelosuppression when a PARP inhibitor is combined with chemotherapy, the first 6 patients accrued were given Veliparib 10 mg bid and TMZ 75 mg/m2/d daily for six weeks. If this was well tolerated, the same doses of Veliparib and TMZ would be tested along with standard radiation with plans to dose escalate the Veliparib in subsequent patient cohorts. Once a maximal tolerated dose was determined, a 78 patient phase II study was planned. Peripheral blood pharmacokinetics were assessed.
Results
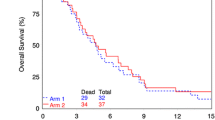
Twenty-four patients were enrolled. In the first 6 patients who received 6 weeks of TMZ with Veliparib only one dose limiting toxicity (DLT) occurred. The next 12 patients received 6 weeks of RT + TMZ + veliparib and 4/12 (33%) had dose limiting hematologic toxicities. As a result, Veliparib was reduced by 50% to 10 mg BID every other week, but again 3/3 patients had dose limiting hematologic toxicities. The trial was then terminated. The mean clearance (± SD) CL/F of Veliparib for the initial dose (27.0 ± 9.0 L/h, n = 16) and at steady-state for 10 mg BID (23.5 ± 10.4 L/h, n = 18) were similar. Accumulation for BID dosing was 56% (± 33%).
Conclusions
Although Veliparib 10 mg BID administered with TMZ 75 mg/m2 for six weeks was well tolerated, when this regimen was combined with standard partial brain irradiation it was severely myelosuppressive even when the dose was reduced by 50%. This study again highlights the potential of localized cranial radiotherapy to significantly increase hematologic toxicity of marginally myelosuppressive systemic therapies.

Similar content being viewed by others
References
CHAMBON P, WEILL JD, MANDEL P (1963) Nicotinamide mononucleotide activation of new DNA-dependent polyadenylic acid synthesizing nuclear enzyme. Biochem Biophys Res Commun 11:39–43. https://doi.org/10.1016/0006-291x(63)90024-x
Chen A (2011) PARP inhibitors: its role in treatment of cancer. Chin J Cancer 30:463–471. https://doi.org/10.5732/cjc.011.10111
Li W, Wang F, Song G, Yu Q, Du R, Xu P (2023) PARP-1: a critical regulator in radioprotection and radiotherapy-mechanisms, challenges, and therapeutic opportunities. Front Pharmacol 14:1198948. https://doi.org/10.3389/fphar.2023.1198948
Shall S, de Murcia G (2000) Poly(ADP-ribose) polymerase-1: what have we learned from the deficient mouse model? Mutat Res 460:1–15. https://doi.org/10.1016/s0921-8777(00)00016-1
Virag L, Szabo C (2002) The therapeutic potential of poly(ADP-ribose) polymerase inhibitors. Pharmacol Rev 54:375–429. https://doi.org/10.1124/pr.54.3.375
Masutani M, Nozaki T, Nakamoto K, Nakagama H, Suzuki H, Kusuoka O, Tsutsumi M, Sugimura T (2000) The response of parp knockout mice against DNA damaging agents. Mutat Res 462:159–166. https://doi.org/10.1016/s1383-5742(00)00033-8
Herrmann GK, Yin YW (2023) The role of poly(ADP-ribose) polymerase 1 in Nuclear and mitochondrial base excision repair. Biomolecules 13:1195. https://doi.org/10.3390/biom13081195
Barazzuol L, Jena R, Burnet NG, Meira LB, Jeynes JCG, Kirkby KJ, Kirkby NF (2013) Evaluation of poly (ADP-ribose) polymerase inhibitor ABT-888 combined with radiotherapy and temozolomide in glioblastoma. Radiat Oncol 8:65–65. https://doi.org/10.1186/1748-717X-8-65
Tisdale MJ (1987) Antitumor imidazotetrazines–XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem Pharmacol 36:457–462. https://doi.org/10.1016/0006-2952(87)90351-0
Denny BJ, Wheelhouse RT, Stevens MF, Tsang LL, Slack JA (1994) NMR and molecular modeling investigation of the mechanism of activation of the antitumor drug temozolomide and its interaction with DNA. Biochemistry 33:9045–9051. https://doi.org/10.1021/bi00197a003
Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, Kros JM, Hainfellner JA, Mason W, Mariani L et al (2005) MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 352:997–1003. 352/10/997 [pii]
Yuan AL, Ricks CB, Bohm AK, Lun X, Maxwell L, Safdar S, Bukhari S, Gerber A, Sayeed W, Bering EA et al (2018) ABT-888 restores sensitivity in temozolomide resistant glioma cells and xenografts. PLoS ONE 13:e0202860. https://doi.org/10.1371/journal.pone.0202860
Wu S, Li X, Gao F, de Groot JF, Koul D, Yung WKA (2021) PARP-mediated PARylation of MGMT is critical to promote repair of temozolomide-induced O6-methylguanine DNA damage in glioblastoma. Neuro Oncol 23:920–931. https://doi.org/10.1093/neuonc/noab003
Tentori L, Portarena I, Torino F, Scerrati M, Navarra P, Graziani G (2002) Poly(ADP-ribose) polymerase inhibitor increases growth inhibition and reduces G(2)/M cell accumulation induced by temozolomide in malignant glioma cells. Glia 40:44–54. https://doi.org/10.1002/glia.10113
Barazzuol L, Jena R, Burnet NG, Jeynes JCG, Merchant MJ, Kirkby KJ, Kirkby NF (2012) In vitro evaluation of combined temozolomide and radiotherapy using X rays and high-linear energy transfer radiation for glioblastoma. Radiat Res 177:651–662. https://doi.org/10.1667/rr2803.1
Lesueur P, Chevalier F, Austry J, Waissi W, Burckel H, Noel G, Habrand J, Saintigny Y, Joly F (2017) Poly-(ADP-ribose)-polymerase inhibitors as radiosensitizers: a systematic review of pre-clinical and clinical human studies. Oncotarget 8:69105–69124. https://doi.org/10.18632/oncotarget.19079
Bhamidipati D, Haro-Silerio JI, Yap TA, Ngoi N (2023) PARP inhibitors: enhancing efficacy through rational combinations. Br J Cancer. https://doi.org/10.1038/s41416-023-02326-7
Coleman RL, Fleming GF, Brady MF, Swisher EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat Ben-Baruch N, Werner TL et al (2019) Veliparib with First-Line Chemotherapy and as maintenance therapy in Ovarian Cancer. N Engl J Med 381:2403–2415. https://doi.org/10.1056/NEJMoa1909707
Han HS, Dieras V, Robson M, Palacova M, Marcom PK, Jager A, Bondarenko I, Citrin D, Campone M, Telli ML et al (2018) Veliparib with temozolomide or carboplatin/paclitaxel versus placebo with carboplatin/paclitaxel in patients with BRCA1/2 locally recurrent/metastatic Breast cancer: randomized phase II study. Ann Oncol 29:154–161. https://doi.org/10.1093/annonc/mdx505
Dieras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub J, Puhalla SL, Bondarenko I, Campone M, Jakobsen EH et al (2020) Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced Breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 21:1269–1282. https://doi.org/10.1016/S1470-2045(20)30447-2
Clarke JM, Patel JD, Robert F, Kio EA, Thara E, Ross Camidge D, Dunbar M, Nuthalapati S, Dinh MH, Bach BA (2021) Veliparib and Nivolumab in combination with platinum doublet chemotherapy in patients with metastatic or advanced non-small cell Lung cancer: a phase 1 dose escalation study. Lung Cancer 161:180–188. https://doi.org/10.1016/j.lungcan.2021.09.004
Tuli R, Shiao SL, Nissen N, Tighiouart M, Kim S, Osipov A, Bryant M, Ristow L, Placencio-Hickok V, Hoffman D et al (2019) A phase 1 study of veliparib, a PARP-1/2 inhibitor, with gemcitabine and radiotherapy in locally advanced Pancreatic cancer. EBioMedicine 40:375–381. https://doi.org/10.1016/j.ebiom.2018.12.060
Czito BG, Deming DA, Jameson GS, Mulcahy MF, Vaghefi H, Dudley MW, Holen KD, DeLuca A, Mittapalli RK, Munasinghe W et al (2017) Safety and tolerability of veliparib combined with capecitabine plus radiotherapy in patients with locally advanced rectal cancer: a phase 1b study. Lancet Gastroenterol Hepatol 2:418–426. https://doi.org/10.1016/S2468-1253(17)30012-2
Donawho CK, Luo Y, Luo Y, Penning TD, Bauch JL, Bouska JJ, Bontcheva-Diaz VD, Cox BF, DeWeese TL, Dillehay LE et al (2007) ABT-888, an orally active poly(ADP-ribose) polymerase inhibitor that potentiates DNA-damaging agents in preclinical Tumor models. Clin Cancer Res 13:2728–2737. https://doi.org/10.1158/1078-0432.CCR-06-3039
Kummar S, Kinders R, Gutierrez ME, Rubinstein L, Parchment RE, Phillips LR, Ji J, Monks A, Low JA, Chen A et al (2009) Phase 0 clinical trial of the poly (ADP-ribose) polymerase inhibitor ABT-888 in patients with advanced malignancies. J Clin Oncol 27:2705–2711. https://doi.org/10.1200/JCO.2008.19.7681
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996. 352/10/987 [pii]
Malanga M, Althaus FR (2005) The role of poly(ADP-ribose) in the DNA damage signaling network. Biochem Cell Biol 83:354–364. https://doi.org/10.1139/o05-038
Dantzer F, Schreiber V, Niedergang C, Trucco C, Flatter E, De La Rubia G, Oliver J, Rolli V, Menissier-de Murcia J, de Murcia G (1999) Involvement of poly(ADP-ribose) polymerase in base excision repair. Biochimie 81:69–75. https://doi.org/10.1016/s0300-9084(99)80040-6
Curtin NJ, Wang L, Yiakouvaki A, Kyle S, Arris CA, Canan-Koch S, Webber SE, Durkacz BW, Calvert HA, Hostomsky Z et al (2004) Novel poly(ADP-ribose) polymerase-1 inhibitor, AG14361, restores sensitivity to temozolomide in mismatch repair-deficient cells. Clin Cancer Res 10:881–889. https://doi.org/10.1158/1078-0432.ccr-1144-3
Wang C, Li J (2021) Haematologic toxicities with PARP inhibitors in cancer patients: an uptodate metaanalysis of 29 randomized controlled trials. J Clin Pharm Ther 46:571–584. https://doi.org/10.1111/jcpt.13349
Farres J, Llacuna L, Martin-Caballero J, Martinez C, Lozano JJ, Ampurdanes C, Lopez-Contreras AJ, Florensa L, Navarro J, Ottina E et al (2015) PARP-2 sustains erythropoiesis in mice by limiting replicative stress in erythroid progenitors. Cell Death Differ 22:1144–1157. https://doi.org/10.1038/cdd.2014.202
Robins HI, Zhang P, Gilbert MR, Chakravarti A, de Groot JF, Grimm SA, Wang F, Lieberman FS, Krauze A, Trotti AM et al (2016) A randomized phase I/II study of ABT-888 in combination with temozolomide in recurrent temozolomide resistant glioblastoma: an NRG oncology RTOG group study. J Neurooncol 126:309–316. https://doi.org/10.1007/s11060-015-1966-z
Sim H, McDonald KL, Lwin Z, Barnes EH, Rosenthal M, Foote MC, Koh E, Back M, Wheeler H, Sulman EP et al (2021) A randomized phase II trial of veliparib, radiotherapy, and temozolomide in patients with unmethylated MGMT glioblastoma: the VERTU study. Neuro Oncol 23:1736–1749. https://doi.org/10.1093/neuonc/noab111
Sarkaria JN, Ballman KV, Kizilbash SH, Sulman EP, Giannini C, Mashru SH, Piccioni DE, Friday BEB, Dixon JG, Kabat B et al (2022) Randomized phase II/III trial of veliparib or placebo in combination with adjuvant temozolomide in newly diagnosed glioblastoma (GBM) patients with MGMT promoter hypermethylation (Alliance A071102). JCO 40:2001. https://doi.org/10.1200/JCO.2022.40.16_suppl.2001
Mehta MP, Wang D, Wang F, Kleinberg L, Brade A, Robins HI, Turaka A, Leahy T, Medina D, Xiong H et al (2015) Veliparib in combination with whole brain radiation therapy in patients with brain metastases: results of a phase 1 study. J Neurooncol 122:409–417. https://doi.org/10.1007/s11060-015-1733-1
Mittapalli RK, Nuthalapati S, Delke DeBord AE, Xiong H (2017) Development of a level A in Vitro-in vivo correlation for Veliparib (ABT-888) extended Release Tablet Formulation. Pharm Res 34:1187–1192. https://doi.org/10.1007/s11095-017-2133-3
Nuthalapati S, Munasinghe W, Giranda V, Xiong H (2018) Clinical pharmacokinetics and Mass Balance of Veliparib in Combination with Temozolomide in subjects with nonhematologic malignancies. Clin Pharmacokinet 57:51–58. https://doi.org/10.1007/s40262-017-0547-z
Singh R, Mehrotra S, Gopalakrishnan M, Gojo I, Karp JE, Greer JM, Chen A, Piekarz R, Kiesel BF, Gobburu J et al (2019) Population pharmacokinetics and exposure-response assessment of veliparib co-administered with temozolomide in patients with myeloid leukemias. Cancer Chemother Pharmacol 83:319–328. https://doi.org/10.1007/s00280-018-3731-4
Gojo I, Beumer JH, Pratz KW, McDevitt MA, Baer MR, Blackford AL, Smith BD, Gore SD, Carraway HE, Showel MM et al (2017) A phase 1 study of the PARP Inhibitor Veliparib in Combination with Temozolomide in Acute Myeloid Leukemia. Clin Cancer Res 23:697–706. https://doi.org/10.1158/1078-0432.CCR-16-0984
Middleton MR, Friedlander P, Hamid O, Daud A, Plummer R, Falotico N, Chyla B, Jiang F, McKeegan E, Mostafa NM et al (2015) Randomized phase II study evaluating veliparib (ABT-888) with temozolomide in patients with metastatic Melanoma. Ann Oncol 26:2173–2179. https://doi.org/10.1093/annonc/mdv308
Kleinberg L, Grossman SA, Piantadosi S, Zeltzman M, Wharam M (1999) The effects of sequential versus concurrent chemotherapy and radiotherapy on survival and toxicity in patients with newly diagnosed high-grade astrocytoma. Int J Radiat Oncol Biol Phys 44:535–543. https://doi.org/10.1016/s0360-3016(99)00060-7
Yovino S, Kleinberg L, Grossman SA, Narayanan M, Ford E (2013) The etiology of treatment-related lymphopenia in patients with malignant gliomas: modeling radiation dose to circulating lymphocytes explains clinical observations and suggests methods of modifying the impact of radiation on immune cells. Cancer Invest 31:140–144. https://doi.org/10.3109/07357907.2012.762780
Kleinberg L, Sloan L, Grossman S, Lim M (2019) Radiotherapy, Lymphopenia, and host Immune Capacity in Glioblastoma: a potentially actionable toxicity Associated with reduced efficacy of Radiotherapy. Neurosurgery 85:441–453. https://doi.org/10.1093/neuros/nyz198
Paganetti H (2023) A review on lymphocyte radiosensitivity and its impact on radiotherapy. Front Oncol 13:1201500. https://doi.org/10.3389/fonc.2023.1201500
Kut C, Kleinberg L (2023) Radiotherapy, Lymphopenia and improving the outcome for glioblastoma: a narrative review. Chin Clin Oncol 12:4–94. https://doi.org/10.21037/cco-22-94
Campian JL, Piotrowski AF, Ye X, Hakim FT, Rose J, Yan X, Lu Y, Gress R, Grossman SA (2017) Serial changes in lymphocyte subsets in patients with newly diagnosed high grade astrocytomas treated with standard radiation and temozolomide. J Neurooncol 135:343–351. https://doi.org/10.1007/s11060-017-2580-z
Hughes MA, Parisi M, Grossman S, Kleinberg L (2005) Primary brain tumors treated with steroids and radiotherapy: low CD4 counts and risk of Infection. Int J Radiat Oncol Biol Phys 62:1423–1426. https://doi.org/10.1016/j.ijrobp.2004.12.085
Funding
This work was supported by Abvie (Abbot Laboratories) and NIH grant UM1 CA 137443 (Adult Brain Tumor Consortium, Principal Investigators: Stuart A. Grossman, Patrick Wen, Burt Nabors).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Lawrence Kleinberg, Xiaobu Ye, Jeff Supko, Glenn Stevens; Hui-Khu Shu; Tom Mikkelson; Frank Lieberman and Glen Lesser. The first draft of the manuscript was written by Emerson Lee and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Financial interests
Dr. Kleinberg reports research support from BMS. Incyte, Novartis, and Novocure as well as honorarium for a study steering committee for Novocure. Dr. Lieberman reports Research funding from Chimerix and Novocure. Dr Grossman, Dr. Stevens, Dr. Mikkelsen, Dr. Ye, Dr. Lesser, and Dr. Supko declare they have no financial interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kleinberg, L., Ye, X., Supko, J. et al. A multi-site phase I trial of Veliparib with standard radiation and temozolomide in patients with newly diagnosed glioblastoma multiforme (GBM). J Neurooncol 165, 499–507 (2023). https://doi.org/10.1007/s11060-023-04514-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-023-04514-0