Abstract
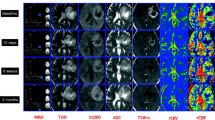
In the context of bevacizumab therapy for the treatment of progressive malignant gliomas, it is currently unclear how different magnetic resonance imaging (MRI) sequences correlate with each other over time. The objective of this study was to determine if a reliable and predictable relationship over time exists between post-gadolinium based contrast agent T1-weighted (T1 + GBCA), T2-weighted, and FLAIR MRI in patients with progressive glioblastoma multiforme (GBM) receiving bevacizumab and chemotherapy. The MRI lesion volumes of 10 patients with progressive GBM that received bevacizumab plus chemotherapy were manually calculated by two independent reviewers. T2 and FLAIR volumes were analyzed by analysis of covariance (ANCOVA) as a function of T1 + GBCA lesion enhancement volume, reviewer, and time interval between MRI acquisitions. Pearson product moment correlation (r) was used to compare pre and post treatment volumes for the group of 10 patients and for each individual patient over their treatment course. ANCOVA demonstrated a significant association between T1 + GBCA and T2-weighted volumes (P = 0.0006) and between T1 + GBCA and FLAIR volumes (P < 0.0001). These associations remained constant over time. The correlation between T1 + GBCA and both T2-weighted and FLAIR volumes improved after bevacizumab treatment. Individual correlations between T1 + GBCA and FLAIR were strong (r ≥ 0.63) with one exception, while correlations between T1 + GBCA and T2 were more variable (r = 0.18–0.99). These findings suggest that FLAIR MRI should be evaluated in addition to T1 + GBCA MRI when evaluating GBM responses.



Similar content being viewed by others
References
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JG (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8:1277–1280
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J, Wick W, Gilbert MR, Lassman AB, Tsien C, Mikkelsen T, Wong ET, Chamberlain MC, Stupp R, Lamborn KR, Vogelbaum MA, van den Bent MJ, Chang SM (2010) Updated response assessment criteria for high-grade gliomas: response assessment in Neuro-Oncology Working Group. J Clin Oncol 28:1963–1972. doi:10.1200/JCO.2009.26.3541
Iwama T, Yamada H, Sakai N, Andoh T, Nakashima T, Hirata T, Funakoshi T (1991) Correlation between magnetic resonance imaging and histopathology of intracranial glioma. Neurol Res 13:48–54
Jahnke K, Muldoon LL, Varallyay CG, Lewin SJ, Brown RD, Kraemer DF, Soussain C, Neuwelt EA (2009) Efficacy and MRI of rituximab and methotrexate treatment in a nude rat model of CNS lymphoma. Neuro Oncology. doi: 10.1215/15228517-2008-119
Russell SM, Elliott R, Forshaw D, Golfinos JG, Nelson PK, Kelly PJ (2009) Glioma vascularity correlates with reduced patient survival and increased malignancy. Surg Neurol. doi: 10.1016/j.surneu.2008.11.012
Batchelor TT, Sorensen AG, di Tomaso E, Zhang WT, Duda DG, Cohen KS, Kozak KR, Cahill DP, Chen PJ, Zhu M, Ancukiewicz M, Mrugala MM, Plotkin S, Drappatz J, Louis DN, Ivy P, Scadden DT, Benner T, Loeffler JS, Wen PY, Jain RK (2007) AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 11:83–95
Varallyay CG, Muldoon LL, Gahramanov S, Wu YJ, Goodman JA, Li X, Pike MM, Neuwelt EA (2009) Dynamic MRI using iron oxide nanoparticles to assess early vascular effects of antiangiogenic versus corticosteroid treatment in a glioma model. J Cereb Blood Flow Metab 29:853–860. doi: 10.1038/jcbfm.2008.162
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Dowell JM, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Wagner M, Bigner DD, Friedman AH, Friedman HS (2007) Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res 13:1253–1259
Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, Ciampa AS, Ebbeling LG, Levy B, Drappatz J, Kesari S, Wen PY (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Jahnke K, Muldoon LL, Varallyay CG, Lewin SJ, Kraemer DF, Neuwelt EA (2009) Bevacizumab and carboplatin increase survival and asymptomatic tumor volume in a glioma model. Neuro Oncology 11:142–150. doi:10.1215/15228517-2008-077
Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U, Curschmann J, Janzer RC, Ludwin SK, Gorlia T, Allgeier A, Lacombe D, Cairncross JG, Eisenhauer E, Mirimanoff RO (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352:987–996
van den Bent MJ, Vogelbaum MA, Wen PY, Macdonald DR, Chang SM (2009) End point assessment in gliomas: novel treatments limit usefulness of classical Macdonald’s criteria. J Clin Oncol. doi: 10.1200/JCO.2009.22.4998
Vredenburgh JJ, Desjardins A, Herndon JE 2nd, Marcello J, Reardon DA, Quinn JA, Rich JN, Sathornsumetee S, Gururangan S, Sampson J, Wagner M, Bailey L, Bigner DD, Friedman AH, Friedman HS (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25:4722–4729
Bokstein F, Shpigel S, Blumenthal DT (2008) Treatment with bevacizumab and irinotecan for recurrent high-grade glial tumors. Cancer 112:2267–2273
Kang TY, Jin T, Elinzano H, Peereboom D (2008) Irinotecan and bevacizumab in progressive primary brain tumors, an evaluation of efficacy and safety. J Neurooncol 89:113–118
Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, Zagzag D, Fischer I, Raza S, Medabalmi P, Eagan P, Gruber ML (2006) Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg 110:173–180
Pope WB, Lai A, Nghiemphu P, Mischel P, Cloughesy TF (2006) MRI in patients with high-grade gliomas treated with bevacizumab and chemotherapy. Neurology 66:1258–1260
Thompson EM, Dosa E, Kreamer DF, Neuwelt E (2010) Treatment with bevacizumab plus carboplatin for recurrent malignant glioma. Neurosurgery 67:87–93
Pronin IN, Holodny AI, Petraikin AV (1997) MRI of high-grade glial tumors: correlation between the degree of contrast enhancement and the volume of surrounding edema. Neuroradiology 39:348–350
Ananthnarayan S, Bahng J, Roring J, Nghiemphu P, Lai A, Cloughesy T, Pope WB (2008) Time course of imaging changes of GBM during extended bevacizumab treatment. J Neurooncol 88:339–347
Gahramanov S, Raslan A, Muldoon LL, Hamilton BE, Rooney WD, Varallyay CG, Njus JM, Haluska M, Neuwelt EA (2010) Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: A pilot study. International J Radiat Oncol Biol Phys. doi:10.1016/j.ijrobp.2009.10.072
Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, Prabhu S, Loghin M, Gilbert MR, Jackson EF. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. doi: 10.1016/j.ijrobp.2009.12.061
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Thompson, E.M., Dosa, E., Kraemer, D.F. et al. Correlation of MRI sequences to assess progressive glioblastoma multiforme treated with bevacizumab. J Neurooncol 103, 353–360 (2011). https://doi.org/10.1007/s11060-010-0397-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0397-0