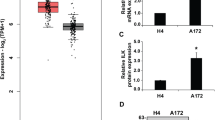
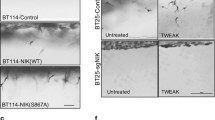
Abstract
Patients afflicted with glioblastoma (GBM) have poor survival due to dispersive invasion throughout the brain. Necl-5, a cell surface receptor for vitronectin, is expressed in GBM but not normal brain. In several GBM cell lines Necl-5 promotes migration and invasion but the mechanism is poorly understood. In this study, we show that knockdown of Necl-5 by RNAi results in markedly decreased invasion of A172 GBM cells in a 3-dimensional matrix. There is a concomitant decrease in the expression and activity of matrix metalloproteinase-2 (MMP-2), a known factor in GBM invasion and disease severity. Knockdown of Necl-5 diminishes basal activation of Akt, an established mediator of MMP-2 expression in gliomas. Knockdown of Necl-5 also limits the maximal Akt activation in response to vitronectin, which requires the activity of Integrin-linked kinase (ILK). During migration, Necl-5, Akt and ILK co-localize at focal contacts at the leading edge of the plasma membrane, suggesting that these molecules may act to integrate Akt signaling at the leading edge to induce MMP-2 expression. By virtue of its restricted expression in GBM and its role in invasion, Necl-5 may be an attractive target for limiting MMP-2 production in glioblastoma, and therefore limiting dispersal.






Similar content being viewed by others
References
Furnari FB, Fenton T, Bachoo RM, Mukasa A, Stommel JM, Stegh A, Hahn WC, Ligon KL, Louis DN, Brennan C, Chin L, DePinho RA, Cavenee WK (2007) Malignant astrocytic glioma: genetics, biology, and paths to treatment. Genes Dev 21:2683–2710
Nakada M, Nakada S, Demuth T, Tran NL, Hoelzinger DB, Berens ME (2007) Molecular targets of glioma invasion. Cell Mol Life Sci 64:458–478
Sloan KE, Eustace BK, Stewart JK, Zehetmeier C, Torella C, Simeone M, Roy JE, Unger C, Louis DN, Ilag LL, Jay DG (2004) CD155/PVR plays a key role in cell motility during tumor cell invasion and migration. BMC Cancer 4:73
Sloan KE, Stewart JK, Treloar AF, Matthews RT, Jay DG (2005) CD155/PVR enhances glioma cell dispersal by regulating adhesion signaling and focal adhesion dynamics. Cancer Res 65:10930–10937
Takai Y, Miyoshi J, Ikeda W, Ogita H (2008) Nectins and nectin-like molecules: roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol 9:603–615
Gromeier M, Lachmann S, Rosenfeld MR, Gutin PH, Wimmer E (2000) Intergeneric poliovirus recombinants for the treatment of malignant glioma. Proc Natl Acad Sci USA 97:6803–6808
Textor S, Durst M, Jansen L, Accardi R, Tommasino M, Trunk MJ, Porgador A, Watzl C, Gissmann L, Cerwenka A (2008) Activating NK cell receptor ligands are differentially expressed during progression to cervical cancer. Int J Cancer 123:2343–2353
Masson D, Jarry A, Baury B, Blanchardie P, Laboisse C, Lustenberger P, Denis MG (2001) Overexpression of the CD155 gene in human colorectal carcinoma. Gut 49:236–240
Erickson BM, Thompson NL, Hixson DC (2006) Tightly regulated induction of the adhesion molecule Necl-5/CD155 during rat liver regeneration and acute liver injury. Hepatology 43:325–334
Ogita H, Ikeda W, Takai Y (2008) Roles of cell adhesion molecules nectin and nectin-like molecule-5 in the regulation of cell movement and proliferation. J Microsc 231:455–465
Giese A, Westphal M (1996) Glioma invasion in the central nervous system. Neurosurgery 39:235–250 (discussion 232–250)
Uhm JH, Dooley NP, Kyritsis AP, Rao JS, Gladson CL (1999) Vitronectin, a glioma-derived extracellular matrix protein, protects tumor cells from apoptotic death. Clin Cancer Res 5:1587–1594
Bafetti LM, Young TN, Itoh Y, Stack MS (1998) Intact vitronectin induces matrix metalloproteinase-2 and tissue inhibitor of metalloproteinases-2 expression and enhanced cellular invasion by melanoma cells. J Biol Chem 273:143–149
Nakada M, Nakamura H, Ikeda E, Fujimoto N, Yamashita J, Sato H, Seiki M, Okada Y (1999) Expression and tissue localization of membrane-type 1, 2, and 3 matrix metalloproteinases in human astrocytic tumors. Am J Pathol 154:417–428
Sawaya RE, Yamamoto M, Gokaslan ZL, Wang SW, Mohanam S, Fuller GN, McCutcheon IE, Stetler-Stevenson WG, Nicolson GL, Rao JS (1996) Expression and localization of 72 kDa type IV collagenase (MMP-2) in human malignant gliomas in vivo. Clin Exp Metastasis 14:35–42
Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PM, Sutherland G, Edwards DR (1999) Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer 79:1828–1835
Price A, Shi Q, Morris D, Wilcox ME, Brasher PM, Rewcastle NB, Shalinsky D, Zou H, Appelt K, Johnston RN, Yong VW, Edwards D, Forsyth P (1999) Marked inhibition of tumor growth in a malignant glioma tumor model by a novel synthetic matrix metalloproteinase inhibitor AG3340. Clin Cancer Res 5:845–854
Tonn JC, Kerkau S, Hanke A, Bouterfa H, Mueller JG, Wagner S, Vince GH, Roosen K (1999) Effect of synthetic matrix-metalloproteinase inhibitors on invasive capacity and proliferation of human malignant gliomas in vitro. Int J Cancer 80:764–772
Noha M, Yoshida D, Watanabe K, Teramoto A (2000) Suppression of cell invasion on human malignant glioma cell lines by a novel matrix-metalloproteinase inhibitor SI-27: in vitro study. J Neurooncol 48:217–223
McCawley LJ, Matrisian LM (2001) Matrix metalloproteinases: they’re not just for matrix anymore!. Curr Opin Cell Biol 13:534–540
Del Duca D, Werbowetski T, Del Maestro RF (2004) Spheroid preparation from hanging drops: characterization of a model of brain tumor invasion. J Neurooncol 67:295–303
Wang M, Wang T, Liu S, Yoshida D, Teramoto A (2003) The expression of matrix metalloproteinase-2 and -9 in human gliomas of different pathological grades. Brain Tumor Pathol 20:65–72
Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y (1994) Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain tumors. J Neurosurg 81:69–77
Sarkar S, Yong VW (2009) Inflammatory cytokine modulation of matrix metalloproteinase expression and invasiveness of glioma cells in a 3-dimensional collagen matrix. J Neurooncol 91:157–164
Heimberger AB, Wang E, McGary EC, Hess KR, Henry VK, Shono T, Cohen Z, Gumin J, Sawaya R, Conrad CA, Lang FF (2005) Mechanisms of action of rapamycin in gliomas. Neurooncology 7:1–11
Lee HC, Park IC, Park MJ, An S, Woo SH, Jin HO, Chung HY, Lee SJ, Gwak HS, Hong YJ, Yoo DH, Rhee CH, Hong SI (2005) Sulindac and its metabolites inhibit invasion of glioblastoma cells via down-regulation of Akt/PKB and MMP-2. J Cell Biochem 94:597–610
Koul D, Shen R, Bergh S, Lu Y, de Groot JF, Liu TJ, Mills GB, Yung WK (2005) Targeting integrin-linked kinase inhibits Akt signaling pathways and decreases tumor progression of human glioblastoma. Mol Cancer Ther 4:1681–1688
Kubiatowski T, Jang T, Lachyankar MB, Salmonsen R, Nabi RR, Quesenberry PJ, Litofsky NS, Ross AH, Recht LD (2001) Association of increased phosphatidylinositol 3-kinase signaling with increased invasiveness and gelatinase activity in malignant gliomas. J Neurosurg 95:480–488
Kleber S, Sancho-Martinez I, Wiestler B, Beisel A, Gieffers C, Hill O, Thiemann M, Mueller W, Sykora J, Kuhn A, Schreglmann N, Letellier E, Zuliani C, Klussmann S, Teodorczyk M, Grone HJ, Ganten TM, Sultmann H, Tuttenberg J, von Deimling A, Regnier-Vigouroux A, Herold-Mende C, Martin-Villalba A (2008) Yes and PI3K bind CD95 to signal invasion of glioblastoma. Cancer Cell 13:235–248
Vlahos CJ, Matter WF, Hui KY, Brown RF (1994) A specific inhibitor of phosphatidylinositol 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002). J Biol Chem 269:5241–5248
Qiao M, Sheng S, Pardee AB (2008) Metastasis and AKT activation. Cell Cycle 7:2991–2996
McDonald PC, Fielding AB, Dedhar S (2008) Integrin-linked kinase—essential roles in physiology and cancer biology. J Cell Sci 121:3121–3132
Edwards LA, Thiessen B, Dragowska WH, Daynard T, Bally MB, Dedhar S (2005) Inhibition of ILK in PTEN-mutant human glioblastomas inhibits PKB/Akt activation, induces apoptosis, and delays tumor growth. Oncogene 24:3596–3605
Shi Q, Bao S, Song L, Wu Q, Bigner DD, Hjelmeland AB, Rich JN (2007) Targeting SPARC expression decreases glioma cellular survival and invasion associated with reduced activities of FAK and ILK kinases. Oncogene 26:4084–4094
Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S (1998) Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc Natl Acad Sci USA 95:11211–11216
Mi Z, Guo H, Wai PY, Gao C, Kuo PC (2006) Integrin-linked kinase regulates osteopontin-dependent MMP-2 and uPA expression to convey metastatic function in murine mammary epithelial cancer cells. Carcinogenesis 27:1134–1145
Li Y, Yang J, Dai C, Wu C, Liu Y (2003) Role for integrin-linked kinase in mediating tubular epithelial to mesenchymal transition and renal interstitial fibrogenesis. J Clin Investig 112:503–516
Chow LM, Baker SJ (2006) PTEN function in normal and neoplastic growth. Cancer Lett 241:184–196
Minami Y, Ikeda W, Kajita M, Fujito T, Amano H, Tamaru Y, Kuramitsu K, Sakamoto Y, Monden M, Takai Y (2007) Necl-5/poliovirus receptor interacts in cis with integrin alphaVbeta3 and regulates its clustering and focal complex formation. J Biol Chem 282:18481–18496
Abe T, Mori T, Kohno K, Seiki M, Hayakawa T, Welgus HG, Hori S, Kuwano M (1994) Expression of 72 kDa type IV collagenase and invasion activity of human glioma cells. Clin Exp Metastasis 12:296–304
Uhm JH, Dooley NP, Villemure JG, Yong VW (1996) Glioma invasion in vitro: regulation by matrix metalloprotease-2 and protein kinase C. Clin Exp Metastasis 14:421–433
Fukushima Y, Tamura M, Nakagawa H, Itoh K (2007) Induction of glioma cell migration by vitronectin in human serum and cerebrospinal fluid. J Neurosurg 107:578–585
Ria R, Vacca A, Ribatti D, Di Raimondo F, Merchionne F, Dammacco F (2002) Alpha(v)beta(3) integrin engagement enhances cell invasiveness in human multiple myeloma. Haematologica 87:836–845
Endersby R, Baker SJ (2008) PTEN signaling in brain: neuropathology and tumorigenesis. Oncogene 27:5416–5430
Koul D, Parthasarathy R, Shen R, Davies MA, Jasser SA, Chintala SK, Rao JS, Sun Y, Benvenisite EN, Liu TJ, Yung WK (2001) Suppression of matrix metalloproteinase-2 gene expression and invasion in human glioma cells by MMAC/PTEN. Oncogene 20:6669–6678
Joy AM, Beaudry CE, Tran NL, Ponce FA, Holz DR, Demuth T, Berens ME (2003) Migrating glioma cells activate the PI3-K pathway and display decreased susceptibility to apoptosis. J Cell Sci 116:4409–4417
Zhang D, Bar-Eli M, Meloche S, Brodt P (2004) Dual regulation of MMP-2 expression by the type 1 insulin-like growth factor receptor: the phosphatidylinositol 3-kinase/Akt and Raf/ERK pathways transmit opposing signals. J Biol Chem 279:19683–19690
Zucker S, Cao J, Chen WT (2000) Critical appraisal of the use of matrix metalloproteinase inhibitors in cancer treatment. Oncogene 19:6642–6650
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25:402–408
Acknowledgments
We thank Jean Stewart for technical assistance. We also thank Drs. Rob Jackson and Alenka Lovy-Wheeler of the Tufts Center for Neuroscience Research (P30 NS047243) for assistance with confocal imaging. The work was funded by grants from the Goldhirsh Foundation and the National Cancer Institute (CA116642).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11060_2010_323_MOESM1_ESM.ppt
Supplemental Fig. S1 HCT116 colon cancer cells were transfected with either control or Necl-5 siRNA (75 nM) using Oligofectamine and following the manufacturer’s directions. Spheroids were made using the hanging drop method as described by Del Duca et al. [21]. Spheroids were implanted into 1.75 mg/ml collagen I and photographed just after implantation (T0) and then 7 days later (T 7ds). Shown are representative micrographs of invasive control and non-invasive Necl-5 depleted spheroids. Nine of each spheroids were implanted, and 9/9 control spheroids were invasive, while 7/9 Necl-5 depleted spheroids were non-invasive. (PPT 22657 kb)
Rights and permissions
About this article
Cite this article
Enloe, B.M., Jay, D.G. Inhibition of Necl-5 (CD155/PVR) reduces glioblastoma dispersal and decreases MMP-2 expression and activity. J Neurooncol 102, 225–235 (2011). https://doi.org/10.1007/s11060-010-0323-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11060-010-0323-5