Abstract
The existence of European ash (Fraxinus excelsior L.) is threatened by fungus-induced ash dieback. It is essential to find effective methods to multiply ash genotypes resistant to ash dieback while preserving the genetic diversity of these tree populations. In this paper the efficient method for production of European ash seedlings using indirect auxiliary organogenesis with multi-factor analysis of its effectiveness is presented. Procedures for a dormancy breaking treatment of seeds and effective disinfection of F. excelsior primary explants, as well as appropriate composition of the culture media taking into account impact of growth regulators and physiological gradient on the micropropagation efficiency were developed. As primary explant for micropropagation of F. excelsior, leaf buds, megagametophytes and zygotic embryos were tested. The best-performing type of primary explant for micropropagation of European ash proved to be zygotic embryos, which were successfully used to regenerate seedlings via indirect auxiliary organogenesis. No statistically significant impact of population origin of F. excelsior explant donor trees was observed on the effectiveness of callus initiation. However, such difference was significant in regard to average productivity of acquired callus cultures (number of seedlings produced) and to average root length of regenerated seedlings. Health condition of explant donor trees and their seeds affects the callus initiation rate from zygotic embryos, but does not affect the productivity of callus lines derived from the seeds and the quality of regenerated seedlings. Indirect auxiliary organogenesis of F. excelsior, developed in our study, not only provides the acquisition of ash seedlings of different genotypes, but also enables rapid selection of desired genotypes already at the callus stage. In this way, the presented method benefits not only profit oriented forestry and wood industry, but also provide the effective and fully controllable tool for reintroduction of various resistant to ash-dieback F. excelsior genotypes without loss of variability and genetic identity of its populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since its first detection in 1992, a chronic fungal disease of European ash (Fraxinus excelsior L.) known as ash dieback, caused by ascomycetous Hymenoscyphus fraxineus (T. Kowalski) Baral et al. (anam. Chalara fraxinea T. Kowalski), has spread throughout numerous countries on several continents (Timmermann et al. 2011; Han et al. 2014; Nielsen et al. 2017). The disease has caused significant economic loss, mostly due to high tree mortality in ash stands reaching up to 85% in plantations and 69% in woodlands (Coker et al. 2019). The amount of damage to ash stands in Europe has become a significant problem as it may disrupt production of ash timber, which is a prized raw material for many industries. Ash wood is coarse-grained, very durable and relatively dense (710 kg/m3) resulting in its high caloric value as firewood. Traditionally, high elasticity of ash wood coupled with its high toughness, that is resistance to propagation of cracks, made it a suitable material in manufacturing of: machine parts including tool handles, especially hammers and axes, bows, paddles, but also billiard or pool table tops, tennis rockets, and various types of gymnastic apparatus. In addition, due to its natural aesthetics, ash wood has been frequently used to produce interior finishing parts for cars, railway passenger cars and planes, as well as parquets and high quality furniture (Hladká 2006; Fraxigen 2005).
There are three Fraxinus species naturally occurring in Europe: European ash (F. excelsior), manna ash (F. ornus) and narrow-leaved ash (F. angustifolia), of which European ash is the most widespread. The species occurs throughout the entire northern Europe up to 64°N parallel (in Norway), reaching the Mediterranean coast to the south and approaching the Volga line to the east. The eastern and southernmost margin of F. excelsior distribution (37° parallel) is located in northern Iran. Two remaining European species have more limited distribution concentrated generally south to the F. excelsior range (Hladká 2006; Dobrowolska et al. 2011). Due to its extensive range F. excelsior is often a key component of many natural forest ecosystems mainly throughout Europe. Thus, its decline caused by ash dieback not only disrupts industries relaying on ash timber, but also poses a significant ecological threat, especially to biodiversity and landscape characteristics of F. excelsior dominated forests. Such forests, affected by massive dieback of ash trees, experience significant decline in numbers of many species more or less dependent on the presence of F. excelsior. The situation is made worse, by frequently observed low quality and/or low yields of F. excelsior seeds. Such seeds germinate erratically making production of high quality seedlings difficult. Moreover, it is the seedlings and young trees that suffer the most form ash dieback, often dying before the first crops of seeds are released (Skovsgaard et al. 2010). Ash dieback caused by the fungus H. fraxineus has been placed on the European and Mediterranean Plant Protection Organization (EPPO) alert list, which includes harmful or pathogenic organisms that pose a pathogenic threat to many plant species including trees. Epidemiological situation in some countries is so difficult that in order to contain the H. fraxineus spread F. excelsior is no longer used in forest renewals (Ioos et al. 2009). The use of fungicides to control the pathogen in forests is not justified, as it would affect the populations of many species reducing biodiversity of forest ecosystem (Queloz et al. 2011; Sieber 2021). Likewise, the removal of infected trees does not offer an effective method to control ash dieback, as it would not reduce significantly the amount of infectious material (i.e. infected leaf rachises and/or petioles) in the forest floor (Coghlan 2012; Kowalski et al. 2015).
On the other hand, susceptibility to ash dieback is genotype-dependent and small fraction of F. excelsior trees in population (usually between 5 and 10%) show high level of natural resistance (Pliura et al. 2011; Menkis et al. 2020). This natural resistance of European ash to ash dieback gives hope that this species will survive under natural conditions (Matiasone et al. 2021). In forestry, the effort to preserve F. excelsior as an economically relevant species relay on effective means to produce high quality seedlings. The most promising method whose widespread application may help to achieve this goal is micropropagation using in vitro cultures, including auxiliary organogenesis, either direct or indirect, and somatic embryogenesis (Shahzad et al. 2017).
Auxiliary organogenesis involves phytohormone-controlled induction of new shoots and roots directly on primary explant (direct organogenesis) or on callus cultures initiated on primary explants (indirect organogenesis) (Phillips and Garda 2019). Somatic embryogenesis, on the other hand, relies on the embryogenic callus, that is, the type of callus able to produce somatic embryos that undergo all the morphogenetic phases characteristic of zygotic embryos (initial stage, globular stage, heart stage, torpedo stage and cotyledonous stage). Both in indirect organogenesis and in somatic embryogenesis, the stage of callus formation is present and this is a main advantage these two techniques over direct organogenesis. Disposal of callus greatly expands the spectrum of laboratory research, especially in plant development biology, biotechnological applications, among others, as a source of valuable secondary metabolites, including pharmaceuticals and cosmetics, but also in conducting dual cultures, especially in phytopathology. Dual cultures can allow e.g. studies of interactions between components of such culture, i.a. in the search for beneficial bioactive factors, but also in pathogen-resistant genotype selection. In these studies, it is important that the callus can be a source of seedlings, is genetically stable, and its maintenance minimizes the risk of mutation and is as little costly as possible. Indirect auxiliary organogenesis facilitates generation callus of these features comparing to somatic embryogenesis. The main advantage of indirect auxiliary organogenesis over somatic embryogenesis method is that it does not involve a difficult and lengthy development of somatic embryos. This results in reduction of the number of necessary passages as regenerated plants are produced on the callus culture itself, and in consequence to lower the cost of expensive media components. What is important, the overall rate of spontaneous mutations is also reduced in this method allowing for the production of more genetically stable seedlings compared to somatic organogenesis (Nawrot-Chorabik 2009).
Therefore, the use of in vitro micropropagation techniques would enable the production of genetically stable seedlings of European ash. What is important, the use of indirect auxiliary organogenesis enables selection of desired genotypes at the callus stage, e.g. genotypes resistant to various biotic and abiotic stresses including the resistance to H. fraxineus infection, and in consequence production of more resistant seedlings. In this study the indirect auxiliary organogenesis of F. excelsior for the effective production of callus and seedling from zygotic embryos is the first time presented. The resulting callus can be used for a variety of laboratory studies including rapid selection of desired genotypes and subsequent seedling production. In addition, multiple seedlings are obtained from a single seed of the desired genotype, which is particularly important in light of the deteriorating seed quality and declining seed yields observed in F. excelsior (Mathews and Rao 1984). Thus, we present the method for the efficient production of F. excelsior seedlings with the desired characteristics. The presented research includes also a comprehensive analysis of the effects of various factors on seedling productivity with the developed method, which can be an effective tool for preventing European ash from dying out without compromising the genetic diversity of its populations.
Materials and methods
Three types of primary explants of F. excelsior, i.e. undeveloped leaf buds, megagametophytes scared with scalpel, and zygotic embryos extracted from mature disinfected seeds were used in the studies.
Undeveloped leaf buds were collected in March 2020. The buds were stored for maximum 3 days at 7 °C ± 1 °C in the dark, humidity c.a. 50%.
Megagametophytes were extracted from immature ash seeds collected in June 2019; at the time of collection the seeds were green, length 25 to 30 mm. The wings of the ash seeds were removed so that only megagametophytes consisting of immature zygotic embryos surrounded with white endosperm remained. Megagametophytes were not stored.
Mature zygotic embryos were extracted from fully-developed ash seeds collected in October 2019 (still before their natural release). The seeds were stored in a ventilated room for maximum 10 months at 7 °C ± 1 °C, in the dark, humidity c.a. 70%. The embryos were extracted from surface-sterilized seeds without wings by incising the seed coat and endosperm.
Seeds from uninfected trees were collected from 45 to 55 year-old F. excelsior growing in Katowice Forest District, Southern Poland (E: 19°01′24″, N: 50°15′51″).
For investigation of the impact of tree age on the effectiveness of disinfection of leaf buds the plant material was collected from F. excelsior, free of fungal infections in three classes of age: young (25–35 year-old), middle-aged (45–55 year-old) and old (60–70 year-old) fruiting trees.
For investigation of the impact of selected phenotype characteristics of trees on the effectiveness of callus initiation and regeneration of seedlings, the plant material, i.e. zygotic embryos, was acquired from mature disinfected seeds collected from F. excelsior trees in four classes of health condition (Table 1). For investigation of the impact of population origin of the explant donor trees on the effectiveness of indirect auxiliary organogenesis, the plant material, i.e. mature seeds, was collected in four Forests Districts: Dukla (E: 21°41′00″, N: 49°33′20″), Jarocin (E: 17°50′19″, N: 51°97′25″), Katowice (E: 19°01′24″, N: 50°15′51″), and Stary Sącz (E: 20°38′05″, N: 49°33′49″).
The efficiency of the callus initiation (callus yield) was calculated as the ratio of number of obtained callus cultures to number of applied zygotic embryos, efficiency of primary seedling formation (callus productivity) was calculated as the ratio of number of primary seedlings to sum of tested callus, while well-rooted secondary seedling yield as the number of seedlings regenerated from a single callus culture. Embryogenic callus formation was verified by Evans blue (EB, 0.1% (w/v)) and Acetocarmine (AC, 2% (w/v)) staining (Guo et al. 2019).
All the experiments were carried out at least in triplicate. Altogether, about 6600 primary explants were used in isolations (at least 100 explants for each experimental combination).
Disinfection of plant material
All the explants were disinfected according to a two-step procedure developed in this study (Table 2).
Development of disinfection method for leaf buds included testing of three modifications of the standard procedure described above (Table 3).
Effectiveness of disinfection procedures was evaluated by culturing of the explants on modified solid Murashige–Skoog (MS) medium for callus initiation and proliferation (Murashige and Skoog 1962, WO2021187995A2) for two weeks at 24 °C ± 1 °C, in the dark and at increased humidity (c.a. 70%).
In vitro culture conditions
All types of primary explants, i.e. disinfected megagametophytes, mature-seeds-extracted zygotic embryos and leaf buds, were cultured in Petri dishes (diam. 90 mm, 4–5 explants per dish) on 0.4% (w/v) agar-solidified MS medium for callus initiation and proliferation (Murashige and Skoog 1962, WO2021187995A2). The cultures were incubated in an air-conditioned plant growth room for 6 weeks at 24 °C ± 1 °C in the dark, and at high humidity (c.a. 70%); after differentiation the cultures were passaged (subculturing) every two weeks. Developed secondary explants (i.e. auxiliary seedlings) cut into two to several fragments (leaves, hypocotyl and root) were transferred onto modified MS medium for indirect auxiliary organogenesis (Murashige and Skoog 1962, WO2021187995A2) and cultured in vitro until morphogenesis under white LED illumination (400–700 nm) (Nawrot-Chorabik 2012) and at relative humidity up to 70%. The regenerated secondary seedlings were passaged into sterile plant culture dishes (diam. 100 mm, height 40 mm–SPL Life Sciences) filled in 2/3 (30 ml) with modified MS medium for rhizogenesis (Murashige and Skoog 1962, WO2021187995A2) and cultured for 3–4 weeks as described above. Then, the rooted seedlings were planted ex vitro in pots filled with sterile standard potting soil.
Similarly after 3 to 4 weeks of culturing, the root length of randomly selected secondary seedlings was measured, and the average root length of seedlings was calculated.
Composition of all the media used in this study, that is, callus initiation and proliferation medium, indirect auxiliary organogenesis medium and rhizogenesis medium, as well as their preparation procedures are covered under patent application no. WO2021187995A2.
Impact of growth regulators on the micropropagation efficiency of F. excelsior via indirect auxiliary organogenesis
This experiment involved culturing of all three types of primary explants: megagametophytes, leaf buds and zygotic embryos extracted from mature seeds, on the modified MS medium (Murashige and Skoog 1962, WO2021187995A2) supplemented with various amounts of growth regulators in two (for callus initiation and proliferation) or three (induction and rooting of primary seedlings formed by the callus) combinations of concentrations (Table 4).
The micropropagation productivity of F. excelsior via indirect auxiliary organogenesis was analyzed by measuring callus and primary seedlings formation efficiency.
Physiological gradient
Physiological gradient in segmentation of fully regenerated secondary seedlings was examined after rhizogenesis. For this, seedlings were dissected into three parts: cotyledon, hypocotyl and root sections that were additionally wounded slightly with scalpel. Such prepared fragments were placed horizontally on the modified MS medium for auxiliary organogenesis (Murashige and Skoog 1962, WO2021187995A2) and cultured under light regime as described above.
Dormancy breaking of seeds (stratification and scarification)
Dormancy breaking procedure involved incubation of mature seeds in gravel-sand substrate (particle size 0.2–0.5 mm) supplemented with 0.05 mg dm−3 (1.44 µM) solution of gibberellic acid (GA3). The seeds were incubated for 10 weeks in the dark at 7 °C in glass bottles (Simax type) filled with the gravel-sand substrate and 50 mL of GA3 solution. The bottles were intensively shaken every 24 h for 5 min. After 10 weeks, the seeds were rinsed to remove particulate residue, subjected to second step of disinfection (Table 2) and used to extract zygotic embryos.
Photosynthesis efficiency measurements of callus and secondary seedlings
The photosynthesis efficiency was measured as maximum quantum efficiency of photosystem II for callus cultures and secondary seedlings regenerated from zygotic embryos extracted from mature seeds collected in Dukla, Jarocin and Katowice Forest Districts. Directly before measurements, the callus cultures and seedlings were kept in dark for 20 min. The measurements were performed with Open FluorCam FC 800−O fluorimeter (Photon Systems Instruments, Brno, Czech Republic) using saturating light pulse, intensity 2700 μE m−2 s−1 for 800 ms.
Statistical analysis
For group comparison, we used the Kruskal–Wallis rank sum test (Hollander et al. 2013). Multiple comparisons after Kruskal–Wallis test were examined with Wilcoxon tests (Hollander et al. 2013) using the compare_means function of the ggpubr package in R environment (version 4.1.0, R Core Team, 2021). P-values lower than 0.05 were considered statistically significant. All the plots were created using ggpubr R package (Kassambara 2020). Error bars represent standard error of the mean.
Results
Disinfection of explants and the impact of explant type on the results of somatic embryogenesis and indirect auxiliary organogenesis in F. excelsior
In this study we tested about 6600 primary explants. Of these, all the megagametophytes and zygotic embryos could be successfully disinfected with 100% efficiency using the developed procedure (Table 2), as no contaminated explants were recorded. For leaf buds, disinfection/preparation procedure tested in this study involved three modifications (Table 3) in which we investigated the impact of bud scales and the tree age on the disinfection effectiveness. The modifications included three age classes, e.g. 25–35, 45–55 and 60–70 year-old and the presence or absence of bud scales. According to our results, procedure no. 3 showed in the best performance in disinfection effectiveness (Fig. 1). Removal of bud scales increased disinfection effectiveness using this procedure for younger and older trees, but did not statistically influenced buds collected from middle-aged trees. This suggests that 45–55 year-old trees may be the best suited for collecting of leaf buds to be used as primary explants, as bud disinfection from these trees does not appear to require time-consuming removal of bud scales. Although leaf buds analyzed in our study could be successfully disinfected, no stable callus line and auxiliary buds were obtained.
Impact of tree age and treatment of undeveloped leaf buds on disinfection efficiency performed according to three procedure modifications described in Table 3
The remaining two types of explants, i.e. megagametophytes and zygotic embryos extracted from mature seeds did produce callus that started to form after approximately 6 weeks of culturing on modified MS medium (Murashige and Skoog 1962, WO2021187995A2). Over 30% of megagametophytes (31.20 ± 4.65%) and nearly 51% of zygotic embryos (50.92 ± 17.17%) initiated callus cultures. After 6 weeks, the average diameter of individual callus cultures reached 8.40 ± 1.20 mm (Fig. 2). Callus initiated on megagametophytes was transparent, partially whitish and well hydrated (Fig. 2A). Combining analysis of cytochemical (EB-AC double staining) and morphological characteristics of the callus showed the embryogenicity of callus produced on megagametophytes—callus cells stained red and formed characteristic centers of prioembryogenic masses (Fig. 2B). Although embryogenic callus was characterized by abundant proliferation it did not form seedlings. After 10 months, the callus became white, compact and less hydrated (Fig. 2C). Ultimately the callus cultures, which were passaged for 21 months, turned brown due to accumulation of phenolic compounds and died.
Morphological and cytochemical analysis of F. excelsior callus cultures initiated on megagametophytes: A phenotype of typical callus (transparent, partially whitish and well hydrated), B centers of proembryogenic masses visible in Evans blue (EB)–Acetocarmine (AC) double staining of the callus and indicative of the embryogenic nature of the callus. Bar = 50 µm), C phenotype of callus after 10 months of culturing (white, compacted and poorly hydrated)
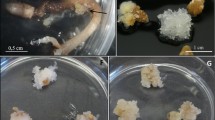
In contrast to megagametophytes, zygotic embryos produced a morphogenetic callus (Fig. 3A), which in the sixth week of culturing starting to produce primary seedlings (Fig. 3B) which, after fragmentation into hypocotyl, cotyledon and root sections, produced secondary seedlings (Fig. 3C, D). This type of callus was light to dark green and was characterized by tough compact structure (Fig. 3A).
Phenotype of morphogenetic callus, primary and secondary seedlings of F. excelsior: A morphogenetic callus initiated on zygotic embryos (light to dark green with compact structure), B primary seedlings formed on the callus, C various primary seedling sections forming secondary seedlings D secondary seedlings formed on various primary seedling sections
Effectiveness of indirect auxiliary organogenesis in relation to population origin of seeds
To verify whether the origin of mature seeds affects callus initiation and regeneration of F. excelsior seedlings via auxiliary organogenesis we examined the seeds collected in four populations. The highest efficiency of the callus initiation, i.e. of 50.91 ± 17.17%, was recorded for zygotic embryos extracted from seeds collected in Katowice Forest District. However, differences between callus yields recorded for the four Forest Districts were not statistically significant (Fig. 4A).
Impact of population origin of seeds on: A efficiency of callus initiation (callus yield) (χ2 = 5.88, df = 3, p = 0.118), B efficiency of primary seedling formation (callus productivity) (χ2 = 9.11, df = 3, p = 0.028), C average root length after rhizogenesis (χ2 = 12.25, df = 3, p = 0.006). P-values from pairwise comparison were placed over horizontal lines
Comparison of primary seedling formation efficiency among four Forest Districts showed that the most productive were the callus cultures initiated from zygotic embryos extracted from seeds collected also in Katowice Forest District (Fig. 4B). This time the differences resulting from the origin of seeds proved to be statistically significant including three out of four Forest Districts (Districts’ pairs: Stary Sącz–Jarocin and Stary Sącz–Dukla). However, no significant differences in the callus productivity were recorded between seeds collected in Katowice and seeds from other Forest Districts (Fig. 4B).
It was also investigated how seed origin affects the root length of secondary seedlings, as this feature is one of the most important factors determining successful acclimatization of the seedlings ex vitro, in soil. The best developed roots were recorded in seedlings acquired from seeds collected in Katowice and Dukla Forest Districts (Fig. 4C). However, the differences in root length between seedlings generated from seeds collected in Dukla and from other Forest Districts were not statistically significant. Interestingly, the longest roots, apart from the seedlings from Katowice Forest District, were found in seedlings from Dukla and Stary Sącz Forest Districts (Fig. 4C). The callus cultures from these Forest Districts were, however, much less productive (Fig. 4B).
Efficiency of indirect auxiliary organogenesis in F. excelsior in relation to content and concentrations of growth regulators
The impact of growth regulators on the micropropagation efficiency in F. excelsior was investigated using three variants of phytohormone concentrations of the basic modified MS medium (Murashige and Skoog 1962, WO2021187995A2) (Table 4). The most efficient callus initiation was achieved using combination 1 (Table 4), that is auxin/cytokinin combination of 0.2 mg × ml−1 of 2,4-dichlorophenoxyacetic acid (2,4-D) and 2 mg × ml−1 6-benzylaminopurine (BA). This combination resulted in over twofold, statistically significant, increase in callus yield compared to combination 2 (Table 5). As for regeneration of primary seedlings, the use of auxin indole-3-butyric acid (IBA) in concentration of 5.28 mg × ml−1 turned out to be the best option as it resulted in statistically significant increase in primary seedling yield compared to abscisic acid (ABA) in concentration of 2.64 mg × ml−1 (Tables 4 and 5).
Impact of seed dormancy breaking treatments on the efficiency of seedling production in F. excelsior via indirect auxiliary organogenesis
The original dormancy breaking method of seeds developed in this study involving combined stratification-scarification treatment (see Materials and Methods) was evaluated for its effectiveness in promoting indirect auxiliary organogenesis in F. excelsior. The method proved to be highly effective. It not only decreased the time required for callus initiation on zygotic embryos by 4 weeks but also increased the productivity of callus cultures. Stratification-scarification treatment resulted in at least 12% of primary explants initiating callus as soon as in the second week of culturing, during the third week the proportion of callus producing explants increased to 52%. However, particularly positive effect of the treatment was observed for production of seedlings, both primary and secondary. During the sixth week of culturing, 19.68% of the callus lines developed with the use of treatment produced secondary seedlings. For the callus lines developed without the stratification-scarification treatment only beginnings of primary seedling production were observed after this time. In addition the treatment facilitated greatly the process of embryos’ extraction from matured F. excelsior seeds.
Physiological gradient
Fully regenerated sterile seedlings after rhizogenesis were evaluated as potential source of secondary explants to increase the ultimate seedling yield. For this, we investigated the physiological gradient of the seedlings and totipotency of their particular parts. The sections, i.e.: cotyledon, hypocotyl and root sections, were used as secondary explants cultured horizontally on the modified MS medium for organogenesis to verify whether they would be able to regenerate into correctly developed seedlings.
The section origin of the secondary explants affected their morphogenesis in all tested seedlings (Fig. 5). Whereas only 5% of hypocotyl secondary explants undergone morphogenesis after 6–8 weeks of culturing, the rate of cotyledon sections that started to produce secondary seedlings reached 70% and the root sections started to produce secondary seedlings indirectly via callus (indirect organogenesis) (Fig. 5).
Effectiveness of callus initiation and seedling production in relation to phenotype characteristics of explant donor trees
In order to verify whether the health condition of trees used as a source of primary explants affects the effectiveness of callus initiation from zygotic embryos and subsequent seedling production we analyzed seeds collected from four F. excelsior trees (Table 1). By far, the best callus initiation yield was recorded for tree no. 4, that is, the tree in the worst health condition, infected by H. fraxineus, and producing small seeds with wings heavily infected by fungal pathogens (Tables 1 and 6).
Also high, but nearly twice lower, callus initiation yields was recorded for trees nos. 2 and 3, i.e. respectively: H. fraxineus-free trees, with well-colored foliage and large seeds infected by fungal pathogens and trees H. fraxineus-infected, with large seeds showing low level of fungal infections on wings (Table 1). The callus initiation rate from healthy trees with large uninfected seeds was nearly 6 times lower than the rate recorded for the tree no. 4. What is more, the callus cultures initiated from the first three trees were less productive compared to cultures acquired from tree no. 4 that appeared healthy, were well hydrated, and fast growing (average weekly mass increase of 7.5 ± 1.1%).
No significant differences were observed in the callus productivity between callus cultures depending on the health condition of the explant donor trees; all callus cultures produced 6 to 8 seedlings.
Measurements of maximum quantum efficiency of photosystem II
The analyzed callus cultures varied statistically in the efficiency of the light-dependent phase of photosynthesis depending on the population origin of the explant donor trees (Fig. 6A). Callus cultures initiated from embryos extracted from seeds collected in Katowice Forest District were characterized by increased quantum efficiency of PSII compared to other Forest Districts what may be related to high callus initiation rated from this material (Fig. 2A). No statistically significant differences in the PSII quantum efficiency of seedlings were recorded (Fig. 6B); the measured values for the seedlings indicate the physiologically correct performance of light-dependent photosynthesis phase.
Impact of population origin of F. excelsior seed donor trees on the maximum quantum efficiency of photosystem II (Fv/Fm): a callus cultures extracted from zygotic embryos (χ2 = 18.20, df = 2, p = 0.000112), b seedlings developed on callus cultures (χ2 = 3.79, df = 2, p = 0.15). P-values from pairwise comparison were placed over horizontal lines
Discussion
Due to the globally widespread extinction of F. excelsior, caused by H. fraxineus, many scientific centers are intensively searching for the effective and environmentally safe tools to save this valuable tree species. One direction of this research is the development of effective methods for propagating trees naturally resistant to the fungal pathogen. Among these methods, those using in vitro culture are of particular interest.
As a result of intensive research, there have been so far many reports indicating that seedling regeneration of several ash species is possible using such in vitro techniques as: axillary shoot formation (Preece et al. 1987; Navarrete et al. 1989) and adventitious shoots induction (Navarrete et al. 1989; Bates et al. 1992; Tabrett and Hammatt 1992; Tonon et al. 2001; Du and Pijut 2008; Palla and Pijut 2011; Stevens and Pijut 2012), somatic embryogenesis (Preece et al. 1989; Bates et al. 1992; Preece and Bates 1995), or regeneration using nodal and apical segments from 13 month to 16-year-old axillary shoots of F. excelsior (Silveira and Cottignies 1994; Nougarède et al. 1996; Schoenweiss and Meier-Dinkel 2005), buds from mature or grafted trees (Hammatt 1994; Nougarède et al. 1996; Pierik and Sprenkels 1997; Thompson et al. 2001; Schoenweiss and Meier-Dinkel 2005), cotyledonary nodes (Hammatt and Ridout 1992), and epicotyls (Mitras et al. 2009), including the selection of types of explants and customization of media and growth regulators content (Dancheva et al. 2013; Dancheva and Iliev 2015). All these studies offer a greater or lesser chance of saving ash trees, but they do not provide a comprehensive tool to simultaneously (i) select genotypes with desirable traits in the laboratory, (ii) research of these genotypes and (iii) efficiently propagate the selected genotypes as callus and seedlings. The most promising method to guarantee all of these above features seems to be indirect auxiliary organogenesis. Results of Mockeliunaite and Kuusiene (2004) described an in vitro experiment using indirect auxiliary organogenesis with F. excelsior zygotic embryos extracted from mature seeds as primary explants. They were used to investigate the impact of light and darkness on the ability of the embryos to produce: hypocotyl, embryonic root, cotyledons and callus. However, the authors were unable to regenerate complete ash seedlings that could be used as planting material. On the other hand Maurizio Capuana (2012) effectively acquired European ash seedlings, but using somatic embryogenesis, which requires more passages than indirect auxiliary organogenesis, what makes it not only more time consuming, but also increases the risk of failure due to contamination inherent to all manipulations of in vitro cultures (Leifert and Cassells 2001; Nawrot-Chorabik 2012, 2016). The lengthy and complicated process of seedling production via somatic embryogenesis results in more opportunities for spontaneous mutations, meaning that seedlings regenerated with this method are not as genetically homogenous as one would expect from a vegetative reproduction method. Moreover, callus is susceptible to chromosomal mutations, including changes to both the number and macrostructure of chromosomes, reducing in time the increasing genome instability and reducing the chance for regeneration of genetically stable seedlings (Bajaj 1986). This problem is not observed in auxiliary organogenesis (Leifert and Cassells 2001; Nawrot-Chorabik 2012, 2016). In the work presented here, indirect auxiliary organogenesis was successfully used to efficient reproduction of F. excelsior including: (i) callus initiation and proliferation, (ii) auxiliary organogenesis and (iii) rhizogenesis of European ash (WO2021187995A2). Among all the primary explants tested, zygotic embryos extracted from mature seeds were the most successful. Although the same type of primary explants and the same micropropagation technique were used by Mockeliunaite and Kuusiene (2004), the modifications introduced in this work provided not only efficient seedling production, but also callus initiation and propagation. Mockeliunaite and Kuusiene (2004) reported that in their experiments the efficiency of callus initiation did not exceed 20%, while our results indicate the efficiency of nearly 60% (Table 6, Fig. 4A).
In order to verify the utility of the method we developed, the influence of selected factors on the micropropagation of F. excelsior was investigated at three stages: (i) callus production by zygotic embryos, (ii) primary seedling formation by callus culture, and (iii) the production of well-rooted secondary seedlings that survived introduction into soil cultures.
One very important factor determining the versatility of the micropropagation method is the origin of the primary explant. The results obtained testify to the versatility of the presented method, as callus, primary seedlings and fully developed secondary seedlings can be obtained regardless of the origin of the zygotic embryos as primary explants. Furthermore, the results obtained show that callus production efficiency does not depend on the origin of the zygotic embryos. This is a favorable characteristics indicating that callus can be easily obtained from different habitats, which is very important at the first level of various laboratory studies on common ash, including the selection of genotypes for the desired trait. On the other hand, callus productivity (numbers of seedlings produced per callus) (Fig. 4B), as well as the degree of rooting of secondary seedlings (Fig. 4C) seem to be more related to the origin of the primary explant. In order to fully verify the validity of this conclusion and to find possible reasons for the observed results, further studies on a large number of zygotic embryos with different population origins are necessary. However, the results obtained for the Katowice Forest District suggest that to maximize the final output of seedlings, the sources of embryos able to initiate cultures with efficient proliferation of the callus are preferable (Fig. 4A). Such callus lines produce more seedlings (Fig. 4B) with better developed roots (Fig. 4C), increasing the probability of successful acclimatization ex vitro. High variability of the callus lines in their seedling output, and maintaining high average callus initiation and average seedling yields with the longest roots, as observed for seeds collected from Katowice Forest District (Fig. 4B), may be considered favorable for practical applications. The more callus cultures initiated, the more genetically diverse callus lines and seedlings are produced and the greater opportunity to select desired genotypes. An important general conclusion from this part of the study is the observation that all of the examined F. excelsior populations could be used as seed donors, resulting in successful callus initiation and regeneration of seedlings. Moreover, the analyzed morphological and physiological parameters of the regenerated seedlings show, that even embryos initiating low-productive callus (Fig. 4A) or callus with low PSII efficiency (Fig. 6A) produce well-developed seedlings, not differing in terms of root system length (Fig. 4C) or the efficiency of the photosynthetic apparatus (Fig. 6B) from the seedling regenerated from high-productive callus lines. This indicates that presented micropropagation procedure is widely applicable enabling to produce well-developed European ash seedlings regardless of the population origin of explant donor trees, although the procedure’s output may vary.
An interesting observation is also the fact that the health condition of the donor tree does not affect the efficiency of seedling production (Table 1), although it does affect the callus initiation yield (Table 6). This last result is intriguing, because it indicated the counter-intuitive relation: i.e. the worse health condition of donor tree, the better callus initiation level (Tables 1 and 6). If the relations were confirmed in further studies, it would indicate that the micropropagation technique via indirect auxiliary organogenesis developed here can be successfully employed even for areas with high ash dieback infection rate, where good-quality primary explants are not readily available. This would enable to effectively renew F. excelsior stands even in the case of poor health condition of the entire population.
We also improved the method of indirect auxiliary organogenesis of F. excelsior using: (i) our developed dormancy breaking of mature seeds prior to embryo extraction, and (ii) a physiological gradient of primary seedlings for their use as secondary explants in micropropagation. The application of the seed dormancy breaking procedure not only greatly facilitated the process of embryo extraction from F. excelsior seeds, but also reduced the callus generation time from 6 to 2 weeks, increased the yield of auxiliary seedlings produced on callus cultures and reduced the time required for full regeneration of secondary seedlings from approximately 10 to 6 weeks. The implementation of a physiological gradient multiplied the efficiency of the procedure, as 70% of root sections of primary seedlings initiated morphogenetic callus capable of producing an additional 6 to 8 secondary seedlings, and approximately 60% of cotyledon sections directly produced seedlings (Fig. 3C, D). In contrast, the tested quantitative and qualitative modifications to the content of growth regulators in the culture medium we developed (WO2021187995A2) did not increase callus initiation efficiency and seedling production (Fig. 5).
One of the key factors determining the success of the application of plant micropropagation techniques is the sterility of the primary explants. The primary explant must be sterile, but the disinfection procedure must not lead to a loss of its regenerative capacity.
In the literature we find several effective methods of disinfection mature ash tree seeds to obtain sterile zygotic embryos as primary explants in a variety of ash micropropagation techniques, although not in seedling production by indirect auxiliary organogenesis (Capuana 2012; Mockeliunaite and Kuusiene 2004). In contrast, no information was found on effective methods to sterilize both megagametophyte and leaf buds of F. excelsior. As the research presented here resulted in sterile zygotic embryos that formed callus capable of producing seedlings with a yield of 6–8 seedlings per callus culture, an attempt was made to develop the effective methods of disinfection of megagametophytes and leaf buds of F. excelsior.
Procedure presented in these studies for the megagametophytes like for mature seeds as a source of zygotic embryos, resulted in not only effective disinfection of explants, but also preserved their ability to initiate the formation of callus. Callus cultures initiated from megagametophytes (Fig. 2) were able to survive approximately 21 months but they did not undergo morphogenesis into seedlings what was observed for callus formed by zygotic embryos (Fig. 3).
Results of experiments aiming to develop an effective disinfection procedure for leaf buds showed that although the best performance was obtained for the buds collected from 45 to 55 year-old trees (Fig. 1), the donor tree age is not a key factor affecting the success rate in disinfection of leaf buds in F. excelsior. The removal of bud scales, on the other hand, proved to be highly effective despite the fact, that instances of better disinfection performance of non-descaled buds were recorded, and disinfection success for buds collected from middle-aged trees did not statistically differ in this matter. A key step of our disinfection procedure of leaf buds appears to be the use of ultrasound treatment in the presence of ascorbic acid (Fig. 1). Ultrasound treatment is one of the more efficient physical techniques used to provide aseptic conditions (Mason 2016). Apart from its mechanical effect, it causes oxidative stress (Miljevic et al. 2014), that on the one hand eliminates microbial contaminants, but on the other damages plant tissues being disinfected. Ascorbic acid, that is an efficient antioxidant, may act as a protective agent for disinfected tissues, as well as to prevent creation of phenols. Thus, designing a disinfection procedure one must reconcile the effectiveness of the sterilizing agent and the need to preserve the vitality of plant tissues. Our procedures allowed the disinfected leaf buds to remain vital for approximately 6 weeks of culturing on the modified MS medium for callus initiation and proliferation (Murashige and Skoog 1962, WO2021187995A20). However, no formation of auxiliary buds were observed. At this time, it is difficult to determine whether undeveloped leaf buds could be used as primary explants for F. excelsior micropropagation. Whether the observed inability of the buds to organogenesis is their natural characteristic or is related to other factors such as the buds’ development stage, remains to be determined. Experiments aiming to investigate this issue are currently underway in our team. Our work focuses also on screening for European ash genotypes resistant to H. fraxineus infection (data not shown). In these successful experiments we generated genetically homogenous callus lines resistant to this pathogen that were able to produce H. fraxineus resistant seedlings. This kind of research confirms practical value of micropropagation methods described in this paper, as it brings hope to reduce the impact of ash dieback without the use of chemical control and genetic engineering methods, maintaining at the same time the full genetic spectrum of European ash as well as genetic identity of its populations.
Conclusion
Our results showed that zygotic embryos are the best type of primary explant, among all the types examined, for seedling regeneration via indirect auxiliary organogenesis in European ash. This assessment included the productivity of the embryo-derived callus cultures themselves, but also the impact of population origin of the explant donor trees, the health condition of donor trees and their seeds and the content and concentrations of growth regulators in the medium. The paper also describes effective disinfection methods for three types of primary explants most often used in micropropagation of trees. Compositions of media suitable for all steps of micropropagation procedure of European ash were also determined. Further, we examined physiological gradient of the regenerated seedlings by evaluation of morphogenetic potential of various seedling sections and their usefulness as secondary explants increasing the micropropagation output. Our results prove the feasibility of micropropagation of European ash via indirect auxiliary organogenesis and its usefulness in reducing the impact of ash decline. The method enables not only the effective production of seedlings from limited amount of plant material, but also selection of callus cultures for desired phenotypes, e.g. resistance to H. fraxineus infection. The produced seedlings are well-rooted increasing their chance for successful acclimatization in natural conditions.
Selected genotypes, carrying preferred breeding traits verified using screening methods enabling fast phenotyping (e.g. measurements of photosynthesis efficiency) (Rousseau et al. 2013; Araus et al. 2018) can be included in breeding projects in forestry, aiming to restore the genetic resources of F. excelsior populations especially heavily affected by ash dieback.
References
Araus JL, Kefauver SC, Zaman-Allah M, Olsen MS, Cairns JE (2018) Translating high-throughput phenotyping into genetic gain. Trends Plant Sci 23(5):451–466. https://doi.org/10.1016/j.tplants.2018.02.001
Bajaj YPS (1986) Biotechnology of tree improvement for rapid propagation and biomass energy production. In: Trees I. Springer, Berlin, pp 1–23. https://doi.org/10.1007/978-3-642-70576-2_1, ISBN 978-3-642-70576-2
Bates S, Preece JE, Navarrete NE, Van Sambeek JW, Gaffney GR (1992) Thidiazuron stimulates shoot organogenesis and somatic embryogenesis in white ash (Fraxinus americana L.). Plant Cell Tiss Org Cult 31:21–29
Capuana M (2012). In vitro propagation of ash (Fraxinus excelsior L.) by somatic embryogenesis. In: Protocols for micropropagation of selected economically-important horticultural plants. Humana Press, Totowa, pp 213–221. https://doi.org/10.1007/978-1-62703-074-8_16
Coghlan A (2012) Are Europe’s ash trees finished? Retrieved 31.10.2012, from the database at http://www.newscientist.com/article/dn22449-are-europes-ash-trees-finished.html
Coker TL, Rozsypálek J, Edwards A, Harwood TP, Butfoy L, Buggs RJ (2019) Estimating mortality rates of European ash (Fraxinus excelsior) under the ash dieback (Hymenoscyphus fraxineus) epidemic. Plants People Planet 1(1):48–58. https://doi.org/10.1002/ppp3.11
Dancheva LD, Iliev I (2015) Factors affecting adventitious shoot formation in Fraxinus excelsior. Propag Ornam Plants 15(1):10–20
Dancheva D, Iliev N, Iliev I (2013) In Vitro Propagation of Fraxinus excelsior L. Oltenia J Stud Nat Sci 29(1). ISSN 1454-6914
Dobrowolska D, Hein S, Oosterbaan A, Wagner S, Clark J, Skovsgaard JP (2011) A review of European ash (Fraxinus excelsior L.): implications for silviculture, For Int J For Res 84(2):133–148. https://doi.org/10.1093/forestry/cpr001
Du N, Pijut PM (2008) Regeneration of plants from Fraxinus pennsylvanica hypocotyls and cotyledons. Sci Hortic 118(1):74–79
Fraxigen (2005) Ash species in Europe: biological characteristics and practical guidelines for sustainable use. University of Oxford, Oxford 128 pp. ISBN: 0 85074 163 7
Guo H-H, Wu J-F, Chen C-X, Wang H-M, Zhao Y-L, Zhang C, Jia YH, Liu F, Ning T-Y, Chu Z-H, Zeng F-C (2019) Identifcation and characterization of cell cultures with various embryogenic/regenerative potential in cotton based on morphological, cytochemical, and cytogenetical assessment. J Integr Agric 18(01):1–8. https://doi.org/10.1016/S2095-3119(17)61876-8
Han JG, Shrestha B, Hosoya T, Lee KH, Sung GH, Shin HD (2014) First report of the ash dieback pathogen Hymenoscyphus fraxineus in Korea. Mycobiology 42(4):391–396. https://doi.org/10.5941/MYCO.2014.42.4.391
Hammatt N (1994) Shoot induction in the leaflet axis of compound leaves from micropropagated shoots of juvenile and mature common ash (Fraxinus excelsior L.). J Exp Bot 45:871–875
Hammatt N, Ridout MS (1992) Micropropagation of common ash (Fraxinus excelsior). Plant Cell Tissue Organ Cult 13:67–74
Hladká D (2006) Ash species in Europe: biological characteristics and practical guidelines for sustainable use. Folia Oecologica 33(2):137
Hollander M, Wolfe DA, Chicken E (2013) Nonparametric statistical methods. John Wiley & Sons, Hoboken
Ioos R, Kowalski T, Husson C, Holdenrieder O (2009). Rapid in planta detection of Chalara fraxinea by a real-time PCR assay using a dual-labelled probe. Eur J Plant Pathol 125(2):329–335. https://doi.org/10.1007/s10658-009-9471-x
Kassambara A (2020) ggpubr: 'ggplot2' Based Publication Ready Plots. R package version 0.4.0. https://CRAN.R-project.org/package=ggpubr
Kowalski T, Bilański P, Holdenrieder O (2015) Virulence of Hymenoscyphus albidus and H. fraxineus on Fraxinus excelsior and F. pennsylvanica. PLoS One 10(10):e0141592. https://doi.org/10.1371/journal.pone.0141592
Leifert C, Cassells AC (2001) Microbial hazards in plant tissue and cell cultures. In Vitro Cell Dev Biol Plant Plant 37(2):133–138. https://doi.org/10.1007/s11627-001-0025-y
Mason TJ (2016) Ultrasonic cleaning: an historical perspective. Ultrason Sonochem 29:519–523. https://doi.org/10.1016/j.ultsonch.2015.05.004. (Epub 2015 May 29. PMID: 26054698)
Mathews VH, Rao PS (1984) In vitro production of multiple seedlings from single seeds of mung bean (Vigna radiata L. Wilczek). Zeitschrift für Pflanzenphysiologie 113(4):325–329. https://doi.org/10.1016/S0044-328X(84)80038-0
Matisone I, Matisons R, Jansons A (2021) The struggle of ash—insights from long-term survey in Latvia. Forests 12(340). https://doi.org/10.3390/f12030340
Menkis A, Bakys R, Åslund MS, Davydenko K, Elfstrand M, Stenlid J, Vasaitis R (2020) Identifying Fraxinus excelsior tolerant to ash dieback: visual field monitoring versus a molecular marker. For Pathol 50:e12572. https://doi.org/10.1111/efp.12572
Miljevic B, Hedayat F, Stevanovic S, Fairfull-Smith KE, Bottle SE, Ristovski ZD (2014) To sonicate or not to sonicate PM filters: reactive oxygen species generation upon ultrasonic irradiation. Aerosol Sci Technol 48(12):1276–1284. https://doi.org/10.1080/02786826.2014.981330
Mitras D, Kitin P, Iliev I, Dancheava D, Scaltsoyiannes A, Tsaktsira M, Nellas CH, Rohr R (2009) In vitro propagation of Fraxinus excelsior L. by epicotyls. J Biol Res Thessaloniki 11:37–48
Mockeliunaite R, Kuusiene S (2004) Organogenesis of Fraxinus excelsior L. by isolated excelsior mature embryo culture. Acta Universitatis Latviensis 676:197–676
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15(3):473–497
Navarrete NE, Van Sambeek JW, Preece JE, Gaffney GR (1989) Improved micropropagation of white ash (Fraxinus americana L.). In: Rink G, Budelsky CA (eds) Proc., 7th central hardwood forest conference. Gen. Tech. Rep. NC-132. USDA For. Serv., St. Paul, MN, pp 146–149
Nawrot‐Chorabik K (2009) Somaclonal variation in embryogenic cultures of silver fir (Abies alba Mill.). Plant Biosyst Int J Deal Aspects Plant Biol 143(2):377–385. https://doi.org/10.1080/11263500902722717
Nawrot-Chorabik K (2012) Somatic embryogenesis in forest plants. In: Embryogenesis, pp 423–446
Nawrot-Chorabik K (2016) Plantlet regeneration through somatic embryogenesis in Nordmann’s fir (Abies nordmanniana). J for Res 27(6):1219–1228. https://doi.org/10.1007/s11676-016-0265-7
Nielsen LR, McKinney LV, Hietala AM, Kjær ED (2017) The susceptibility of Asian, European and North American Fraxinus species to the ash dieback pathogen Hymenoscyphus fraxineus reflects their phylogenetic history. Eur J for Res 136(1):59–73. https://doi.org/10.1007/s10342-016-1009-0
Nougarède A, Silveira CE, Rondet P (1996) In nature dormant buds and in vitro dormant-like buds of Fraxinus excelsior L. Protoplasma 190:16–24
Palla KJ, Pijut PM (2011) Regeneration of plants from Fraxinus americana hypocotyls and cotyledons. In Vitro Cell Dev Biol Plant 47:250–256
Pierik RLM, Sprenkels PA (1997) Micropropagation of Fraxinus excelsior L. (common ash). In: Bajaj YPS (ed) Biotechnology in agriculture and forestry. Trees V, vol 39. Springer, Berlin
Phillips GC, Garda M (2019) Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol Plant 55(3):242–257. https://doi.org/10.1007/s11627-019-09983-5
Pliura A, Lygis V, Suchockas V, Bartkevicius E (2011) Performance of twenty four European Fraxinus excelsior populations in three Lithuanian progeny trials with a special emphasis on resistance to Chalara fraxinea. Balt for 17(1):17–34
Preece JE, Bates S (1995) Somatic embryogenesis in white ash (Fraxinus americana L.). In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants, vol 2. Kluwer Acad. Publ., Dordecht, pp 311–325
Preece JE, Christ PH, Ensenberger L, Zhao JI (1987) Micropropagation of ash (Fraxinus). Comb Proc Int Plant Prop Soc 37:366–372
Preece JE, Zhao JI, Kung FH (1989) Callus production and somatic embryogenesis from white ash. Hort Sci 24:377–380
Queloz V, Grünig CR, Berndt R, Kowalski T, Sieber TN, Holdenrieder O (2011) Cryptic speciation in Hymenoscyphus albidus. For Pathol 41(2):133–142. https://doi.org/10.1111/j.1439-0329.2010.00645.x
Rousseau C, Belin E, Bove E, Rousseau D, Fabre F, Berruyer R et al (2013) High throughput quantitative phenotyping of plant resistance using chlorophyll fluorescence image analysis. Plant Methods 9(1):17
Schoenweiss K, Meier-Dinkel A (2005) In vitro propagation of selected mature trees and juvenile embryo-derived cultures of Common ash (Fraxinus excelsior L.). Propag Ornam Plants 5:137–145
Shahzad A, Sharma S, Parveen S, Saeed T, Shaheen A, Akhtar R et al (2017) Historical perspective and basic principles of plant tissue culture. In: Plant biotechnology: principles and applications. Springer, Singapore, pp 1–36. https://doi.org/10.1007/978-981-10-2961-5_1
Sieber TN (2021) The phyllosphere mycobiome of woody plants. In: Forest microbiology. Academic Press, pp 111–132. https://doi.org/10.1016/B978-0-12-822542-4.00003-6
Silveira CE, Cottignies A (1994) Period of harvest, sprouting ability of cuttings, and in vitro plant regeneration in Fraxinus excelsior. Can J Bot 72(2):261–267. https://doi.org/10.1139/b94-035
Skovsgaard JP, Thomsen IM, Skovgaard IM, Martinussen T (2010) Associations among symptoms of dieback in even‐aged stands of ash (Fraxinus excelsior L.). For Pathol 40(1):7–18. https://doi.org/10.1111/j.1439-0329.2009.00599.x
Stevens ME, Pijut PM (2012) Agrobacterium-mediated transformation and regeneration of Pumpkin Ash (Fraxinus profunda) hypocotyls. In Vitro Cell Dev Biol Plant Anim 48:39–40
Tabrett AM, Hammatt N (1992) Regeneration of shoots from embryo hypocotyls of common ash (Fraxinus excelsior). Plant Cell Rep 11:514–518
Timmermann V, Børja I, Hietala AM, Kirisits T, Solheim H (2011) Ash dieback: pathogen spread and diurnal patterns of ascospore dispersal, with special emphasis on Norway. EPPO Bulletin 41(1):14–20. https://doi.org/10.1111/j.1365-2338.2010.02429.x
Tonon G, Capuana M, Di Marco A (2001) Plant regeneration of Fraxinus angustifolia by in vitro shoot organogenesis. Sci Hort 87:291–301
Thompson D, Harrington F, Douglas G, Hennerty M, Nakhshab N, Long R (2001) Vegetative propagation techniques for oak, ash, sycamore and spruce. COFORD Publ, Dublin
Patents
WO2021187995A2 Worldwide Patent Latowski, D., Nawrot-Chorabik, K. The method of obtaining saplings of the common ash (Fraxinius excelsior L.) and the media suitable for use in this method https://worldwide.espacenet.com/patent/search/family/075954228/publication/WO2021187995A2?q=PCT%2FPL2021%2F050017. Accessed on 23 September 2021.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Nawrot-Chorabik, K., Pluciński, B. & Latowski, D. Indirect auxiliary organogenesis of Fraxinus excelsior L. as a tool for ash dieback control. New Forests 55, 323–344 (2024). https://doi.org/10.1007/s11056-023-09981-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-023-09981-x