Abstract
Our research demonstrates that plant material can be produced in the nursery with asymmetrical root systems, which may have utility for reforestation of difficult planting sites characterized by steep slopes and/or windy conditions. Such a root system can be generated using chemical root pruning by applying cupric carbonate (Cu) that can arrest the development of, or cause mortality to, root apical meristems resulting in the formation of new lateral roots with an overall increase in the biomass, length, and volume of the root system. Our objective was to investigate the effect of chemical root pruning on the morphological and architectural traits of adventitious roots produced by poplar cuttings (Populus nigra L.) grown in containers coated with Cu in various symmetrical (Side, Bottom, Side + Bottom) and asymmetrical (half side + half bottom) patterns. After six weeks, roots of the cuttings were extracted from different container depths (Top, Middle, and Bottom) and portions (non-coated, Cu-coated), and analyzed. The root systems reacted to all coating patterns by increasing length, biomass, volume, and average diameters, but magnitude of increase was further affected by depth. In particular, root growth was unaffected at the Top of the container, and length was the highest at the Bottom depth. The Middle depth had a significant increment in both biomass and volume. Also, the root population increased in diameter as a possible response to Cu exposure. Interestingly, in the asymmetrically coated containers this depth response in the non-coated portions was of higher magnitude than in the Cu-coated portions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Asymmetrical copper root pruning may result in the modulation of the root traits improving the plant material performance for reforesting sites with peculiar conditions (e.g., steep slope, dominant wind).
Introduction
Both short- and long-term change of climate are considered important drivers of forest degradation (Stanturf et al. 2014), increasing the need for restoration across all scales (Chen et al. 2011). In turn, global forest restoration initiatives are fundamental for reducing atmospheric concentration of carbon dioxide and mitigating climate change (Bastin et al. 2019). Forest restoration strategies can be achieved by many techniques and tools (Stanturf et al. 2014) and among these, container seedlings are a cost-effective alternative, especially when the planting season is to be extended or adverse sites are to be planted (Stanturf et al. 2014; Montagnoli et al. 2016; Löf et al. 2019).
For nursery seedlings, the Target Plant Concept is a means for defining the characteristics desired in plants that address limiting factors on the outplanting site so that desired field survival and growth is achieved, i.e., the seedlings have “fitness for purpose” (Landis and Dumroese 2006; Luoranen et al. 2006; Wang et al. 2007; Landis et al. 2010a, b; Cole et al. 2011; Dumroese et al. 2016; Montagnoli et al. 2018). Potential limiting factors may include steep slopes, wind, and their interaction that increases susceptibility to mechanical forces that tend to topple trees, especially when the crowns of recently outplanted seedlings have grown to intercept appreciable wind during storms (Quine et al. 2007; Sung et al. 2010; Hale et al. 2012; Haywood et al. 2012; James et al. 2014; Dumroese et al. 2019; Montagnoli et al. 2020). And, this scenario is expected to worsen because changes in climate have increased the number and intensity of windstorms affecting forests (Dale et al. 2001; Seidl et al. 2017).
Plant anchorage is the primary function of coarse roots and permits plant survival, but poor architectural characteristics of the root system can lead to anchorage failure (Haywood et al. 2012; Yang et al. 2017). Trees exposed to mechanical forces on steep slopes and/or prevailing wind conditions respond by developing a specific asymmetrical root architecture. This increases tree stability by modifying the distribution of mechanical forces in the soil Danjon et al. 2005; Lombardi et al. 2017; Dumroese et al. 2019; Montagnoli et al. 2019, 2020; Deljouei et al. 2020). In particular, these root systems exhibit strong selective leeward and/or windward reinforcements (Pinus pinaster, Danjon et al. 2005; Pinus ponderosa, Dumroese et al. 2019) and may display the most root length and root volume either upslope (Quercus pubescens, Di Iorio et al. 2005; Spartium junceum, Lombardi et al. 2017) or downslope (Pinus ponderosa, Dumroese et al. 2019). These asymmetrical architectures are very different from those observables in nurseries when poor management leads to seedlings too big for their containers (e.g., excessive fertilization or retaining the seedlings too long in the containers) resulting in plants with spiraling or matted root systems (e.g., Landis et al. 2010a, b; Dumroese and Landis 2015). Poor root system quality adversely affects post-planting growth performance and mechanical stability (Fernández et al. 2007; Sayer et al. 2009; Liu et al. 2016). Instead, it might be assumed that the induction of an asymmetrical root distribution during seedling production in a container nursery may ensure a greater anchorage and a better establishment potential once outplanted. Therefore, how nursery-grown container seedlings will perform and establish on slopes with prevailing wind is of special concern for reforestation activities, and root traits affecting tree stability should be considered in breeding programs (Telewski and Moore 2016).
Copper (Cu) root pruning is a cultural practice in nurseries. Many forms of Cu, including cupric carbonate (CuCO3), have been used to coat container walls. Cupric carbonate treatments in the nursery influence the abundance and distribution of roots after outplanting, which, in turn, improves stem stability (Krasowski 2003) and other seedling performance attributes (Burdett et al. 1986; Mexal et al. 1991). Copper is an essential metal involved in many proteins important for plant growth and development. However, when present in excess, Cu can lead to inhibition of root elongation, disturbance of mitosis, and damage of root epidermal cells and cell membranes (Arduini et al. 1995; Jiang et al. 2001; Sheldon and Menzies 2005; Qin et al. 2015). Therefore, when Cu is used to coat the inner container walls, a growing lateral root that contacts Cu halts its growth and the injury-stimulated response is to produce more higher-order roots (Xu et al. 2017; Baesso et al. 2018) that yields a more fibrous root system (Wenny et al. 1988; Gilman and Beeson 1995; Sayer et al. 2009). Thus, Cu root pruning is effective in reducing spiraling and caging (Ruehle 1985; Wenny and Wollen 1989) because roots no longer grow downward along the container wall–substrate interface (Wenny et al. 1988; Sayer et al. 2011; Haywood et al. 2012). In addition, Cu root pruning seems to improve root growth potential (South et al. 2005; Haywood et al. 2012). All studies conducted so far focused, however, on a symmetrical distribution of Cu inside the container leading to roots being more evenly distributed (Wenny and Wollen 1989; Gilman and Beeson 1995). In the present study, we hypothesized that an asymmetrical distribution of Cu within the container would generate an asymmetrical root system. If so, such a root system might have potential for improving seedling performance after outplanting on sloped sites and/or with a dominant wind direction. To test our hypothesis, we applied a latex (water-based) paint solution containing 100 g L− 1 of CuCO3 in various combinations of the interior surfaces of containers used to grow poplar (Populus nigra L.) cuttings. After six weeks, cuttings were sampled and six root traits (biomass, length, volume, mean diameter, tissue density, and specific root length) were analyzed according to different sectors (i.e., depth: Top, Middle, and Bottom) of the container.
Materials and methods
Treatment, plant material, growth room characteristics, and growing conditions
To explore symmetrical and asymmetrical application of Cu on rooting, we used truncated-cone-shaped plastic containers (Research Centre for Forestry and Wood patent n° 1236/A/87) having a top diameter of Ø 10 cm, a bottom diameter of Ø 7.5 cm, a total height of 16.5 cm, and a volume of 1 L. A white, water-based paint solution containing 100 g L− 1 CuCO3 was applied symmetrically (in respect to the centered vertical axis of the container) to three surfaces of the container (Side, Bottom, or Side + Bottom). The same Cu solution was applied asymmetrically (i.e., vertically to just one half of the Side + Bottom). Containers without the paint-Cu solution were the Control (Fig. 1) (Wenny et al. 1988).
Top view of containers showing different combinations of copper applications to the interior container surface. Below each photograph is a schematic view of the different container sectors analyzed (Top, Middle, Bottom). Portions with the same color reflect results presented in Fig. 3 (symmetrical Cu-coated) and Fig. 4 (asymmetrical Cu-coated)
Each container was filled with a commercial soil-less substrate characterized by 1:2:1 (v:v:v) mixture of peat, silica sand, and bark humus. The basal 6–7 cm of a single 20-cm-long, 1.7-cm-basal diameter poplar cutting provided by the Research Council for Agricultural Research and Economic Analysis (CREA; Casale Monferrato, AL, Italy) was struck into each container. We employed 10 replicates; thus, 50 containers in total, 3 symmetrical treatments (Side, Bottom, Side + Bottom), 1 asymmetrical treatment (Side + Bottom), and 1 Control. We used a single (100-cm wide, 130-cm deep, adjustable height from 70 to 120-cm) growth chamber at the University of Insubria, Varese, Italy. The chamber was illuminated with fluorescent light (Fluora T8 (OSRAM); LEDVANCE GmbH; Garching, Germany). Cuttings were grown with long day conditions (16 h light/8 h dark cycle) under 22/17°C day/night temperature and air humidity maintained at 60–70%. Light intensity yielded approximately 300 µmol m− 2 s− 1 (Light Meter HD2302.0; Delta Ohm; Caselle di Selvazzano, Italy) at the plants’ top and was kept constant during their growth by the adjustment of the chamber floor. Irrigation frequency was determined gravimetrically: we first watered the soil medium until saturation, allowed the substrate to drain to container capacity, measured that initial mass, and then subsequently irrigated back to container capacity each time container mass reached 60% of initial mass (Dumroese et al. 2015).
Seedling sampling and analysis
After 6 weeks of growth, cuttings were gently pulled from each container to ensure the root plug remained intact. For the symmetrical Cu-coated containers, root plugs were transversally cut into three sectors based on depth: 0-4.5 cm (Top), 4.5–9.8 cm (Middle), and 9.8–16.5 cm (Bottom) (Fig. 1). Because of container taper, each sector had the same volume: 333 cm3. Root plugs from asymmetrical Cu-coated containers were also transversally cut at the same three depths, but each root plug sector was further cut in half longitudinally (Cu and no-Cu), separating a total of six portions (Fig. 1). For the Control containers, the root plug was divided the same as the asymmetrical Cu-coated containers.
The original cutting, both above and below ground, together with branches were considered as stem. Leaves were detached from branches. Each subdivision of the root plug was gently washed over a 2 mm sieve. All roots from each cutting were collected and then scanned (600 dpi) with a calibrated flatbed scanner coupled to a lighting system for image acquisition (Epson Expression 10,000 XL). Length (m), diameter (mm), and volume (cm3) of roots were measured using WinRhizo Pro V. 2007d (Regent Instruments Inc. Quebec, Canada). Separate stem, leaf, and root fractions were oven dried at 75 °C until constant weight to obtain biomass (g) values. Morphometric data together with biomass data were used to calculate the relative morphological traits of specific root length (m g− 1) and root tissue density (g cm− 3).
Statistical analysis
A one-way ANOVA was performed on stem, leaves, total root biomass, and root-to-shoot ratio data to test the effect of Cu treatments (Control, symmetrical and asymmetrical treated containers). Post hoc Bonferroni tests were conducted to detect overall differences among treatments.
For the symmetrical containers, a General Linear Model (GLM) repeated measures was performed considering, as among factors, the Cu treatment (Control, Side, Bottom, Side + Bottom) and, as within factors, the sector (Top, Middle, Bottom) on root biomass, volume, length, diameter, specific root length, and root tissue density. In particular, in order to compare Control and treated plants, data from the two portions of each sector of Control plants were pooled together before performing the statistical analysis. Post hoc Bonferroni tests were conducted among estimated marginal means to detect overall differences among Cu treatment within the same sector.
For the asymmetrical Cu-coated containers, a GLM repeated measures was performed considering, as among factors, the Cu treatment (Control, Asymmetrical) and, as within factors, the six portions (Top, Middle, Bottom / no-Cu and Cu sides) on root biomass, volume, length, diameter, specific root length, and root tissue density. Post hoc Bonferroni tests were used to test differences among the portions of each Cu treatment, and the same portions between the Cu treatments.
When needed to ensure normal distributions and equal variances for ANOVA and symmetrical and asymmetrical GLM models, the dependent variables were square-root transformed. Differences were considered significant at p < 0.05. Statistical analysis was computed with SPSS 25.0 (SPSS Inc, Chicago IL, USA).
Results
General Cu vs. no Cu
Biomass and root-to-shoot ratio
Stem biomass was similar among Control and Cu-treated cuttings with one exception: cuttings grown in Side + Bottom symmetrical Cu-coated containers had significantly less biomass (Fig. 2 A). Leaf biomass was unaffected by the treatments. Root biomass was more variable. Control cuttings had the lowest root biomass whereas cuttings exposed to Bottom or asymmetrical Cu-applications had the most. Cuttings exposed to Side and Side + Bottom showed an intermediate response (Fig. 2 A). The root-to-shoot ratio for Control cuttings was significantly lower than that of cuttings grown with Side + Bottom or asymmetrical Cu-applications whereas Side and Bottom cuttings had an intermediate response (Fig. 2B).
Stem, leaf, and root biomass (A) and root-to-shoot ratio (B) for different combinations of copper (CuCO3) applications. Vertical boxes represent 50% of the observations (25th to 75th percentiles) and lines extending from each box are the upper and lower 25% of the distribution (90th and 10th percentiles). Within each box, the solid horizontal line is the mean value and the dotted line is the median. Means with different letters (i.e., a and b for stems and x and y for roots) indicate significant differences among treatments (Bonferroni test, p < 0.05)
Symmetrical Cu-coated pots: root traits according to depths
Biomass
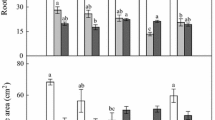
The general linear model (GLM) repeated measures performed for symmetrical application of copper within container walls showed that the sector (i.e., depth - D; Top, Middle, and Bottom), the Cu treatment (C; Control, Side, Bottom, and Side + Bottom), and their interaction (D x C), significantly affected the root biomass (Table 1). No significant differences were detected in the Top depth regardless of which surfaces were treated with Cu (Fig. 3 A). In the Middle depth, Control cuttings had the least biomass whereas those exposed to the Bottom application had the most, with cuttings in the Side and Side + Bottom applications intermediate in response (Fig. 3 A). Bottom depth results were mostly similar to those of the Middle depth (Fig. 3 A).
Root biomass (A), length (B), volume (C), diameter (D), tissue density (E), and specific root length (F) of cuttings grown in containers receiving symmetrical application of cupric carbonate (CuCO3) to their Side, Bottom, or Side + Bottom. Vertical boxes represent 50% of the observations (25th to 75th percentiles) and lines extending from each box are the upper and lower 25% of the distribution (90th and 10th percentiles). Within each box, the solid horizontal line is the mean value and the dotted line is the median. Means with different letters (i.e., a and b or x, y, and z) indicate significant differences among treatments within each depth (Bonferroni test, p < 0.05)
Length
The presence of Cu alone did not affect the root length, while the sector factor both alone and in combination with Cu factor did (Table 1). Root length of Control and Cu-treated cuttings was not significantly different in the Top or Middle depths, but in the Bottom the Control cuttings had the shortest length, whereas Bottom and Side + Bottom cuttings had the most, with Side cuttings intermediate in their response (Fig. 3B).
Volume
As for the biomass, the GLM repeated measures showed that the sector, the Cu treatment, and their interaction, significantly affected the root volume (Table 1). No treatment differences were seen in the Top depth, whereas in the Bottom the Control cuttings had significantly less volume than any of the Cu treatments (Fig. 3 C). The Middle depth was intermediate; Control cuttings had the least volume, Side cuttings the most, with Bottom and Side + Bottom treatments not significantly different than the Control or Side.
Mean diameter
The sector factor alone significantly affected the root diameter while the presence of Cu both alone and in combination with the sector did not (Table 1). Mean diameter of roots was not differing between the three depths (Top, Middle, and Bottom) independent of the treatment (Fig. 3D). However, we observed a trend toward all Cu treatments having higher diameter compared to the control, athough the diameters were not significantly different (Fig. 3D).
Root tissue density
The sector factor alone significantly affected the root tissue density while the presence of Cu both alone and in combination with the sector did not (Table 1). No interaction of depth and Cu was observed (Fig. 3E). Regardless of depth, root tissue density was not significantly different among the Control or Cu applications (Fig. 3E).
Specific root length
Specific root length was unaffected by Cu, depth, or their interaction (Table 1; Fig. 3 F).
Asymmetrical Cu-coated pots: root traits according to portions
Biomass
The portion factor alone did not affect the root biomass while the presence of Cu both alone and in combination with the portion did (Table 2). In asymmetrical Cu-coated pots, biomass in the no-Cu portions of the Middle and Bottom depths was significantly higher than in the Top depth as well as for the same portions in the Control pots (Fig. 4 A). Biomass on the Cu-treated side was similar among the three depths, and the Bottom depth had significantly more biomass than the same depth in the Control containers (Fig. 4 A).
Root biomass (A), length (B), volume (C), diameter (D), tissue density (E), and specific root length (F) of Control and asymmetrical Cu-coated pots. Vertical boxes represent 50% of the observations (25th to 75th percentiles) and lines extending from each box are the upper and lower 25% of the distribution (90th and 10th percentiles). Within each box, the solid horizontal line is the mean value and the dotted line is the median. Circles represent values less than the 10th percentile or greater than the 90th percentile. Means with different letters (i.e., a and b for control containers and x, y, and z for asymmetrical containers) indicate significant differences among portions within each Cu-treatment (Bonferroni test, p < 0.05). Asterisks (*) indicate significant difference (p < 0.05) within the same portions and between Cu treatment and control seedlings
Length
The portion factor alone did not affect the root length, while the presence of Cu both alone and in combination with the portion did (Table 2). In asymmetrical Cu-coated pots, the no-Cu Bottom portion had significantly higher length values compared to the Top depth, with the Middle depth intermediate in response (Fig. 4B). Moreover, both Middle and Bottom no-Cu portions had significantly higher length values than did the corresponding portions of the Control plants (Fig. 4B). Length on the Cu-treated side was similar among the three depths and with the same portions of Control plants with the only exception being the bottom depth, which was significantly higher (Fig. 4B).
Volume
The portion factor alone did not affect the root volume, while the presence of Cu both alone and in combination with the portion did (Table 2). In asymmetrical Cu-coated pots, the no-Cu Middle and Bottom depths had significantly higher volume than that of the Top depth (Fig. 4 C), as well as significantly higher values than in the same depths for the Cu and Control portions (Fig. 4 C). Moreover, in the Cu-treated Bottom depth, volume was higher than the Top depth and the corresponding portion of Control cuttings (Fig. 4 C).
Mean diameter
The portion factor alone significantly affected the root diameter, while the presence of Cu alone did not (Table 2). Also, the combination of portion and Cu significantly affected the mean diameter (Table 2). For Control cuttings, the mean diameter decreased moving downward from Top to Bottom (Fig. 4D). Differently, diameter measured for cuttings grown in the no-Cu side of asymmetrical Cu-coated pots was the highest in the Middle, lowest in the Top, and intermediate in the Bottom although these differences were not significant (Fig. 4D). Moreover, root diameter in the no-Cu and Cu portions were similar to those in the corresponding portions for Control plants with the only exception of the Bottom depth in the no-Cu portion (Fig. 4D).
Root tissue density
Both the portion and the copper factor alone and their combination did not affect the root tissue density (Table 2). Also, the combination of portion and Cu significantly affected the mean diameter (Table 2). Moreover, no significant differences were observed between Control and asymmetrically Cu-treated cuttings or among portions (Fig. 4E).
Specific root length
The specific root length was significantly affected by the combination of portion and copper, while these factors alone were not significant (Table 2). For Control cuttings, specific root length increased moving downward from Top to Bottom, whereas the addition of Cu to one side of the container resulted in no significant differences among depths or with Control plants (Fig. 4 F). In the top and bottom depth, no-Cu side in the asymmetrical Cu-treated cuttings had respectively higher and lower specific root length compared with the Control cuttings (Fig. 4 F).
Discussion
In our study, the comparison of Populus nigra cuttings grown without Cu or with Cu applied symmetrically or asymmetrically indicated that after 6 weeks of growth the Cu application generally reduced shoot biomass and increased root biomass. However, this modification in the biomass partitioning was of different magnitude among the various combinations of Cu applications. In particular, stem biomass of treated cuttings was significantly lower than that of Control cuttings only when Cu was applied symmetrically to the entire inner surface (i.e., Side + Bottom). This result concurs with a review that reported a decrease in shoot growth of different species with increasing amounts of root pruning (Geisler and Ferree 2011). Thus, in our study it is reasonable that Cu application to the entire interior surface of the container may correspond to the highest level of root pruning, which leads to the greater reduction of shoot growth. However, other research regarding the variation of stem biomass in relation to Cu application is not consistent. Some researchers report a lack of effect on aboveground plant growth (Dunn et al. 1997; Sayer et al. 2009) whereas sometimes average shoot height and dry weight were improved (Burdett and Martin 1982; McDonald et al. 1984; Ruehle 1985; Aldrete et al. 2002; Barnett and McGilvray 2002; Tsakaldimi and Ganatsas 2006). The inconsistency between our findings and other reported results might be related to the plant material used. Indeed, poplar cuttings have a peculiar early developmental pattern of the root and shoot (Branislav et al. 2009). New root growth is largely supported by current assimilates, while stored assimilates are mostly used for shoot growth (Pregitzer and Friend 1996). In our study, it would be possible to suppose that once the above ground fraction has used the stored assimilates to develop, the rate of shoot growth would decrease favoring development of the Cu-stimulated root system. Indeed, root biomass was generally higher for treated cuttings, and increased significantly when Cu was applied partially to the inner surface of containers (Bottom and asymmetrically). The general increase of the root-to-shoot ratio we observed supports this differentiated response.
These growth-partitioning responses might be related to the functional equilibrium of biomass allocation, that is, when plants allocate relatively more biomass to a specific organ (i.e., roots or shoots) depending on whether the limiting factor for growth is below- (e.g., nutrients, water, predatory activity), or above- (e.g., light, CO2) ground (Brouwer 1963; Thornley 1972; Iwasa and Roughgarden 1984; Poorter et al. 2012). In this respect, plants having part of their leaves or roots removed show remarkable resilience in that they restore allocation patterns quickly to reach the pre-damaged levels (Brouwer 1963; Poorter and Nagel 2000; Poorter et al. 2012). Similarly, in our case the root biomass increment may be related to the direct effect of Cu, which inhibits root tip growth thereby increasing secondary root branching at the expense of shoot growth (Arnold and Struve 1993; Arduini et al. 1995; Crawford 1997) and underlining a possible diversified effect on primary and secondary root tissues, the former being more susceptible to the Cu effect. Therefore, it appears that a plant’s reaction to Cu is to adjust its inner balance by increasing root growth and, thus, directing more current assimilates to the root system. The analysis of root traits according to the different depths for symmetrically Cu-coated containers (i.e., Top, Middle, and Bottom) and the different portions of asymmetrically Cu-coated containers (i.e., no-Cu, Cu) revealed that our methodological approach was effective in controlling root elongation of poplar cuttings. The level of effectiveness, however, followed a common pattern among different combinations of copper application to the interior surfaces of the containers. Interestingly, we observed a lack of influence on root traits in the Top depth, the most biomass and volume in the Middle, and the longest root length in the Bottom depth.
These depth differences may be related to substrate moisture content. In any container, a perched water table forms at the bottom of the container immediately after irrigation because of the cohesive nature of water (Landis et al. 2014). Thus, the substrate at the bottom of the container remains moister longer than the substrate at the top of the container, where evaporation accelerates drying (Argo and Biernbaum 1994). In Populus, formation of adventitious roots (i.e. plant roots that form from any non-root tissue) is enhanced by increased substrate moisture content (Puri and Thompson 2003), so more roots may have formed in the lower, moister substrate profile than in the upper, drier profile. This effect may have been exacerbated given that cuttings often generate most roots at their base, which in this study would have been positioned lower in the container and in substrate that stayed moisture longer. Once formed, Populus roots further respond to increased substrate moist by increasing growth (Zhao et al. 2014). In addition, the longer duration of hypoxic conditions in the Bottom depth may have generated a lower Cu concentration with subsequent relief of the Cu exposure-effect to roots (Crawford 2003; Marler and Musser 2016) and/or the effectiveness of Cu is lower when substrates are saturated, similar to the observation that Cu availability as a plant nutrient is reduced when substrates are saturated (Sims and Patrick 1977; Vogel and Jokela 2011). Finally, longer roots in the Bottom could be a function of stress; growth of adventitious roots is recognized as a response to stress conditions, including flooding, nutrient deprivation, and wounding (Steffens and Rasmussen 2016). In our case, the saturated soil condition occurring at the Bottom depth of the container could be a stressful condition comparable to flooding occurring in nature. Thus, a possible explanation of the observed differences by depth in our study is the occurrence of variation in water content at different container depths that cause a complex interplay of these factors. An irrigation regime with less amplitude in the level of drying (compared to the regime used in this study that had more amplitude because of the drier irrigation target) and/or a different substrate having physical properties that promote more uniform moisture levels, may yield a more uniform response during nursery production. Understanding this contrasting root development pattern in relation to container depth is relevant when considering future success of outplanted seedlings in field conditions because roots in the upper and lower soil profiles are known to be fundamental for seedling stability (Montagnoli et al. 2020) and resource uptake (Pierret et al. 2016), respectively.
Interestingly, our results showed that Cu application at a rate of 100 mg L− 1 induced an increase in the diameter size of the root population. Probably at this concentration and for our studied species, plant material, and experimental conditions, Cu inhibits the root apical meristems stopping the longitudinal root growth in favor of the radial growth type and the emission of new larger roots (Montagnoli et al. 2014, 2021; Amendola et al. 2017). In particular, the enlargement due to the radial growth has been seen in response to physical interruption of the root apex (Montagnoli et al. 2014) and also in response to the decreased need of exploring nutrient and water search versus the need of a more structured root system devoted to reserve accumulation (Amendola et al. 2017; Montagnoli et al. 2021). We may speculate that the root population became thicker in reaction to Cu exposure as adaptation to such a toxic environment. Indeed, Vitis labrusca L. plantlets grown in pots with high Cu soil contents had larger root diameters due to the increase of the cortex area, which may contribute to a lower absorption and higher retention of Cu in the roots, impairing its transport to the shoots where it may cause severe damage (Ambrosini et al. 2015, 2018). Moreover, the production of new lateral roots of larger diameter might be explained by the need of the plants to replace the removed or injured apex (Stokes et al. 2009). Important is that these roots have a lower tissue density than Control ones, indicating that new root production was fast for a productive growth species like poplar, which leads to investment in cheap tissue promoting fast growth with probably shorter life span (Kramer-Walter et al. 2016). Our findings also concur with the non-linear relationship between root diameter and root tissue density, which were inversely related for woody species (Kong et al. 2019). Once again, these differences where detectable independently of the combination of Cu application to the different portions of the root plug, indicating a clear systemic response to local applications of Cu.
In the case of asymmetrically Cu-coated containers, although the Cu side showed higher values than Control for some of the measured traits (i.e., biomass, length, and volume in the Cu Bottom portion), increments of higher magnitude in respect to the Control were found in the untreated side (i.e., biomass, length, and volume in both Middle and Bottom portion). Furthermore, no differences between treated and untreated portions were measured for the mean diameter of the root population clearly indicating that the root lengthening was responsible for the increment of the volume and biomass. This differentiated response of the root system has already been observed in split-root system experiments, which provides a way to simulate the heterogeneity inherent to field conditions (Fernández et al. 2021). Finally, also in this case, our results indicate both a local and systemic response of the root system to the local Cu application. In particular, the higher response was observed in the opposite side of the Cu application indicating a possible autoregulation, probably controlled by the shoot, to maintain the rooting pattern. These results support our initial hypothesis that an asymmetrical distribution of Cu within the container would lead to an asymmetrical root system. Our findings highlight for the first time the possibility to have plants with an asymmetrical root distribution that, in the short-to-medium term, could provide a more stable tree cover on sloped and windy sites. Our study provides foundation for future research that better unveils the response of root systems to asymmetrical chemical pruning and subsequent root architecture development on steep and/or windy sites.
Conclusions
Our findings highlighted the possibility to gather specific root traits and information on localized root development when Cu was applied to discrete portions of the inner surfaces of containers. We found it possible to modulate the Cu to optimize different areas of the root system. The induction of these modifications on the plant material that is destinated to sites with peculiar conditions (e.g., steep slope, dominant wind direction) would potentially improve their performance once outplanted. Moreover, the poplar cuttings seem to respond at the whole system level to a local, discrete application of Cu. Finally, root chemical pruning due to the Cu application seems to increase the size of the root population resulting in a root system longer and larger with a lower tissue density, which may have a longer life span.
Availability of data and material
The data presented in this study are available on request from the corresponding author.
References
Aldrete A, Mexal JG, Phillips R, Vallotton AD (2002) Copper coated polybags improve seedling morphology for two nursery-grown Mexican pine species. For Ecol Manag 163:197–204. DOI: https://doi.org/10.1016/S0378-1127(01)00579-5
Ambrosini VG, Rosa DJ, Prado JPC, Borghezan M, Melo GWB, Soares CRFS, Comin JJ, Simão DG, Brunetto G (2015) Reduction of copper phytotoxicity by liming: a study of the root anatomy of young vines (Vitis labrusca L.). Plant Physiol Biochem 96:270–280. DOI: https://doi.org/10.1016/j.plaphy.2015.08.012
Ambrosini VG, Rosa DJ, Bastos de Melo GW, Zalamena J, Cella C, Guimarães Simão D, Souza da Silva L, Pessoa dos Santos H, Toselli M, Tiecher TL, Brunetto G (2018) High copper content in vineyard soils promotes modifications in photosynthetic parameters and morphological changes in the root system of ‘Red Niagara’ plantlets. Plant Physiol Biochem 128:89–98. DOI: https://doi.org/10.1016/j.plaphy.2018.05.011
Amendola C, Montagnoli A, Terzaghi M, Trupiano D, Oliva F, Barontic S, Migliettac F, Chiatante D, Scippa GS (2017) Short-term effects of biochar on grapevine fine root dynamics and arbuscular mycorrhizae production. Agric Ecosyst Environ 239:236–245. DOI: https://doi.org/10.1016/j.agee.2017.01.025
Arduini I, Godbold DL, Onnis A (1995) Influence of copper on root growth and morphology of Pinus pinea L. and Pinus pinaster Ait. seedlings. Tree Physiol 15:411–415. DOI: https://doi.org/10.1093/treephys/15.6.411
Argo WR, Biernbaum JA (1994) Irrigation requirements, root-medium pH, and nutrient concentrations of Easter lilies grown in five peat-based media with and without an evaporation barrier. J Amer Soc Hort Sci 119:1151–1156. DOI: https://doi.org/10.21273/JASHS.119.6.1151
Arnold MA, Struve DK (1993) Root distribution and mineral uptake of coarse-rooted trees grown in cupric hydroxide-treated containers. HortScience 28:988–992
Baesso B, Chiatante D, Terzaghi M, Zenga D, Nieminen K, Mahonen AP, Siligato R, Helariutta Y, Scippa GS, Montagnoli A (2018) Transcription factors PRE3 and WOX11 are involved in the formation of new lateral roots from secondary growth taproot in A. thaliana. Plant Biol 20:426–432. DOI: https://doi.org/10.1111/plb.12711
Barnett JP, McGilvray JM (2002) Copper-treated containers influence root development of longleaf pine seedlings. In: Barnett JP, Dumroese RK, Moorhead DJ (eds) Proceedings of workshops on growing longleaf pine in containers—1999 and 2001. Gen Tech Rep SRS-56. USDA Forest Service, Southern Research Station, Asheville, NC, pp 24–26
Bastin J-F, Finegold Y, Garcia C, Mollicone D, Rezende M, Routh D, Zohner CM, Crowther TW (2019) The global tree restoration potential. Science 365:76–79. DOI: https://doi.org/10.1126/science.aax0848
Branislav K, Savo R, Dragana M, Petar I, Marina K (2009) Early shoot and root growth dynamics as indicators for the survival of black poplar cuttings. New For 38:177–185. DOI https://doi.org/10.1007/s11056-009-9138-7
Brouwer R (1963) Some aspects of the equilibrium between overground and underground plant parts. Jaarboek van het Instituut voor Biologisch en Scheikundig onderzoek aan Landbouwgewassen, pp 31–39
Burdett AN, Coates H, Eremko R, Martin PAF (1986) Toppling in British Columbia’s lodgepole pine plantations: significance, cause and prevention. For Chron 62:433–439. DOI: https://doi.org/10.5558/tfc62433-5
Burdett AN, Martin PAF (1982) Chemical root pruning of coniferous seedlings. HortScience 16:622–624
Chen I-C, Hill JK, Ohlemüller R, Roy DB, Thomas CD (2011) Rapid range shifts of species associated with high levels of climate warming. Science 333:1024. DOI: https://doi.org/10.1126/science.1206432
Cole RJ, Holl KD, Keene C, Zahawi RA (2011) Direct seeding of late-successional trees to restore tropical montane forest. For Ecol Manag 261:1590–1597. DOI: https://doi.org/10.1016/j.foreco.2010.06.038
Crawford MA (1997) Update on copper root control. In: Landis TD, Thompson JR (eds) National proceedings, forest and conservation nursery associations. Gen Tech Rep PNW- GTR-419. USDA Forest Service, Pacific Northwest Research Station, Portland, OR, pp 120–124
Crawford MA (2003) Copper-coated containers and their impact on the environment. In: Riley LE, Dumroese RK, Landis TD (eds) National proceedings. forest and conservation nursery associations–2002. RMRS-P-28. USDA Forest Service, Rocky Mountain Research Station, Fort Collins, CO, pp 76–78
Dale VH, Joyce LA, McNulty S, Neilson RP, Ayres M, Flannigan MD, Hanson PJ, Irland LC, Lugo AE, Peterson CJ, Simberloff D, Swanson FJ, Stocks BJ, Wotton BM (2001) Climate change and forest disturbances: climate change can affect forests by altering the frequency, intensity, duration, and timing of fire, drought, introduces species, insect and pathogen outbreaks, hurricanes, windstorms, ice storms, or landslides. BioScience 51:723–734. DOI: https://doi.org/10.1641/0006- 3568(2001)051[0723:CCAFD]2.0.CO;2
Danjon F, Fourcaud T, Bert D (2005) Root architecture and wind-firmness of mature Pinus pinaster. New Phytol 168:387–400. DOI: https://doi.org/10.1111/j.1469-8137. 2005.01497.x
Deljouei A, Abdi E, Schwarz M, Majnounian B, Sohrabi H, Dumroese RK (2020) Mechanical characteristics of the fine roots of two broadleaved tree species from the temperate Caspian Hyracanian Ecoregion. Forests 11:345. DOI: https://doi.org/10.3390/f11030345
Di Iorio A, Lasserre B, Scippa GS, Chiatante D (2005) Root system of Quercus pubescens trees growing on different sloping conditions. Ann Bot 95:351–361. DOI: https://doi.org/10.1093/aob/mci033
Dumroese RK, Landis TD (2015) Growing container seedlings: three considerations. Tree Planters’ Notes 58(2):58–62
Dumroese RK, Landis TD, Pinto JR, Haase DL, Wilkinson KW, Davis AS (2016) Meeting forest restoration challenges: using the target plant concept. Reforesta 1:37–52. DOI: https://doi.org/10.21750/REFOR.1.03.3
Dumroese RK, Terzaghi M, Chiatante D, Scippa GS, Lasserre B, Montagnoli A (2019) Functional traits of Pinus ponderosa coarse-roots in response to slope conditions. Front Plant Sci 10:947. DOI: https://doi.org/10.3389/fpls.2019.00947
Dunn GM, Huth JR, Lewty MJ (1997) Coating nursery containers with copper carbonate improves root morphology of five native Australian tree species used in agroforestry systems. Agroforest Syst 37:143–155. DOI: https://doi.org/10.1023/A:1005863707277
Fernández IS, Černý M, Skalák J, Brzobohatý B (2021) Split root systems: detailed methodology, alternative applications, and implications at leaf proteome level. Plant Methods 17:7. DOI: https://doi.org/10.1186/s13007020 00706 1
Fernández M, Tejero JR, Pérez I, Soria F, Ruiz F, López G (2007) Effect of copper coating nursery containers on plant growth and root morphology of Eucalyptus globulus Labill. cuttings and seedlings. Silva Lusitana 15:215–227
Geisler D, Ferree DC (2011) Response of plants to root pruning. Hortic Reviews 6:155–188. DOI: https://doi.org/10.1002/9781118060797.ch5
Gilman EF, Beeson RJ (1995) Copper hydroxide affects root distribution of Ilex cassine in plastic containers. HortTechnology 5:48–49. DOI: https://doi.org/10.21273/HORTTECH.5.1.48
Hale SE, Gardiner BA, Wellpott A, Nicoll BC, Achim A (2012) Wind loading of trees: influence of tree size and competition. Eur J For Res 131:203–217. DOI: https://doi.org/10.1007/s10342-010-0448-2
Haywood JD, Sung SS, Sword Sayer MA (2012) Copper root pruning and container cavity size influence longleaf pine growth through five growing seasons. South J Appl For 36:146–151. DOI: https://doi.org/10.5849/sjaf.10-051
Iwasa Y, Roughgarden J (1984) Shoot/root balance of plants: optimal growth of a system with many vegetative organs. Theor Popul Biol 25:78–105. DOI: https://doi.org/10.1016/0040-5809(84)90007-8
James KR, Dahle GA, Grabosky J, Kane B, Detter A (2014) Tree biomechanics literature review: dynamics. Arbor Urban For 40:1–15. DOI: https://doi.org/10.48044/jauf.2014.001
Jiang W, Liu D, Liu X (2001) Effect of copper on root growth, cell division, and nucleolus of Zea mays. Biol Plant 44:105–109. DOI: https://doi.org/10.1023/A:1017982607493
Kong D, Wang J, Wu H, Valverde-Barrantes OJ, Wang R, Zeng H, Kardol P, Zhang H, Feng Y (2019) Nonlinearity of root trait relationships and the root economics spectrum. Nat Commun 10:2203. DOI: https://doi.org/10.1038/s41467-019-10245
Kramer-Walter KR, Bellingham PJ, Millar TR, Smissen RD, Richardson SJ, Laughlin DC (2016) Root traits are multidimensional: specific root length is independent from root tissue density and the plant economic spectrum. J Ecol 104:1299–1310. DOI: https://doi.org/10.1111/1365-2745.12562
Krasowski MJ (2003) Root system modifications by nursery culture reflect on post-planting growth and development of coniferous seedlings. For Chron 79:882–891. DOI: https://doi.org/10.5558/tfc79882-5
Landis TD, Dumroese RK (2006) Applying the target plant concept to nursery stock quality. In: MacLennan L, Fennessy J (eds) Plant quality: a key to success in forest establishment. National Council Forest Research and Development, Dublin, Ireland, pp 1–10
Landis TD, Dumroese RK, Haase DL (2010a) The Target Plant Concept. In: Landis TD, Dumroese RK, Haase DL (eds) Container tree nursery manual vol 7: seedling processing, storage, and outplanting. Agric Handbk 674. USDA Forest Service, Washington, DC, pp 3–15
Landis TD, Luna T, Dumroese RK (2014) Containers, Chap. 7. In: Wilkinson KM, Landis TD, Haase DL, Daley BF, Dumroese RK (eds) Tropical nursery manual: a guide to starting and operating a nursery for native and traditional plants. Agric Handbk 732. USDA Forest Service, Washington, DC, pp 123–139
Landis TD, Steinfeld DE, Dumroese RK (2010b) Native plant containers for restoration projects. Native Plants J 11:341–348. DOI: 10.1353/npj.2010b.0006
Liu J, Bloomberg M, Li G, Liu Y (2016) Effects of copper root pruning and radicle pruning on first-season field growth and nutrient status of Chinese cork oak seedlings. New For 47:715–729. DOI: https://doi.org/10.1007/s11056-016-9540-x
Löf M, Madsen P, Metslaid M, Witzell J, Jacobs DF (2019) Restoring forests: regeneration and ecosystem function for the future. New For 50:139–151. DOI: https://doi.org/10.1007/s11056-019-09713-0
Lombardi F, Scippa GS, Lasserre B, Montagnoli A, Tognetti R, Marchetti M, Chiatante D (2017) The influence of slope on Spartium junceum root system: morphological, anatomical and biomechanical adaptation. J Plant Res 130:515–525. DOI: https://doi.org/10.1007/s10265-017-0919-3
Luoranen J, Rikala R, Konttinen K, Smolander H (2006) Summer planting of Picea abies container-grown seedlings: Effects of planting date on survival, height growth and root egress. For Ecol Manag 237:534–544. DOI: https://doi.org/10.1016/j.foreco.2006.09.073
Marler T, Musser C (2016) Chemical and air pruning of roots influence post-transplant root traits of the critically endangered Serianthes nelsonii. Plant Root 10:21–25. DOI:https://doi.org/10.3117/plantroot.10.21
McDonald SE, Tinus RW, Reid CPP (1984) Modification of ponderosa pine root systems in containers. J Environ Hort 2:1–5. DOI: https://doi.org/10.24266/0738-2898-2.1.1
Mexal JG, South DB (1991) Bareroot seedling culture. In: Duryea ML, Dougherty PM (eds) Forest regeneration manual. Kluwer Academic Publishers, The Netherlands, pp 89–115. DOI: https://doi.org/10.1007/978-94-011-3800-0_6
Montagnoli A, Baronti S, Alberto D, Chiatante D, Scippa GS, Terzaghi M (2021) Pioneer and fibrous root seasonal dynamics of Vitis vinifera L. are affected by biochar application to a low fertility soil: a rhizobox approach. Sci Total Environ 751:141455. DOI: https://doi.org/10.1016/j.scitotenv.2020.141455
Montagnoli A, Dumroese RK, Terzaghi M, Pinto JR, Fulgaro N, Scippa GS, Chiatante D (2018) Tree seedling response to LED spectra: implications for forest restoration. Plant Biosyst 152:515–523. DOI: https://doi.org/10.1080/11263504.2018.1435583
Montagnoli A, Lasserre B, Sferra G, Chiatante D, Scippa GSS, Terzaghi M, Dumroese RK (2020) Formation of annual ring eccentricity in coarse roots within the root cage of Pinus ponderosa growing on slopes. Plants 9:181. DOI:https://doi.org/10.3390/plants9020181
Montagnoli A, Terzaghi M, Chiatante D, Scippa GS, Lasserre B, Dumroese RK (2019) Ongoing modifications to root system architecture of Pinus ponderosa growing on a sloped site revealed by tree-ring analysis. Dendrochronologia 58:125650. DOI: https://doi.org/10.1016/j.dendro.2019.125650
Montagnoli A, Terzaghi M, Fulgaro N, Stoew B, Wipenmyr J, Ilver D, Rusu C, Scippa GS, Chiatante D (2016) Non-destructive phenotypic analysis of early stage tree seedling growth using an automated stereovision imaging method. Front Plant Sci 7:1644. DOI: https://doi.org/10.3389/fpls.2016.01644
Montagnoli A, Terzaghi M, Scippa GS, Chiatante D (2014) Heterorhizy can lead to underestimation of fine-root production when using mesh-based techniques. Acta Oecol 59:84e90. DOI: https://doi.org/10.1016/j.actao.2014.06.004
Pierret A, Maeght J-L, Clément C, Montoroi J-P, Hartmann C, Gonkhamdee S (2016) Understanding deep roots and their functions in ecosystems: an advocacy for more unconventional research. Ann Bot 118:621–635. DOI: https://doi.org/10.1093/aob/mcw130
Poorter H, Nagel O (2000) The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: a quantitative review. Aust J Plant Physiol 27:595–607. DOI: https://doi.org/10.1071/PP99173_CO
Poorter H, Niklas KJ, Reich PB, Oleksyn J, Poot P, Mommer L (2012) Biomass allocation to leaves, stems and roots. meta-analyses of interspecific variation and environmental control New Phytol 193:30–50. DOI: https://doi.org/10.1111/j.1469-8137.2011.03952.x
Pregitzer KS, Friend AL (1996) The structure and function of Populus root systems. In: Stettler RF, Bradshaw HD, Heilman PE, Hinckley TM (eds) Biology of Populus and its implications for management and conservation. NRC Research Press, Ottawa, Canada, pp 331–353
Puri S, Thompson F (2003) Relationship of water to adventitious rooting in stem cuttings of Populus species. Agroforest Syst 58:1–9. DOI: https://doi.org/10.1023/A:1025494221846
Qin R, Wang C, Chen D, Björn LO, Li S (2015) Copper-induced root growth inhibition of Allium cepa var. Agrorarum L. involves disturbances in cell division and DNA damage. Environ Toxicol Chem 34:1045–1055. DOI: https://doi.org/10.1002/etc.2884
Quine CP, Gardiner BA (2007) Understanding how the interaction of wind and trees results in windthrow, stem breakage and canopy gap formation. In: Johnson E, Miyanishi K (eds) Plant disturbance ecology: the process and the response. Elsevier Academic Press, Amsterdam, pp 103–156
Ruehle JL (1985) The effect of cupric carbonate on root morphology of containerized mycorrhizal pine seedlings. Can J Forest Res 15:586–592. DOI: https://doi.org/10.1139/x85-095
Sayer MAS, Haywood JD, Sung S-JS (2009) Cavity size and copper root pruning affect production and establishment of container-grown longleaf pine seedlings. For Sci 55:377–389. DOI: https://doi.org/10.1093/forestscience/55.5.377
Sayer MAS, Sung S-JS, Haywood JD (2011) Longleaf pine root system development and seedling quality in response to copper root pruning and cavity size. South J Appl For 35:5–11. DOI: https://doi.org/10.1093/sjaf/35.1.5
Seidl R, Dominik T, Kautz M, Martin-Benito D, Peltoniemi M, Vacchiano G, Wild J, Ascoli D, Petr M, Honkaniemi J, Lexer MJ, Trotsiuk V, Mairota P, Svoboda M, Fabrika M, Nagel TA, Reyer (2017) Forest disturbances under climate change. Nat Clim Change 7:395–402. DOI: https://doi.org/10.1038/nclimate3303
Sheldon AR, Menzies NW (2005) The effect of copper toxicity on the growth and root morphology of Rhodesgrass (Chloris gayana Knuth.) in resin buffered solution culture. Plant Soil 278:341–349. DOI https://doi.org/10.1007/s11104-005-8815-3
Sims JL, Patrick WH Jr (1977) The distribution of micronutrient cations in soil under conditions of varying redox potential and pH. Soil Sci Soc Am J 42:258–262. DOI: https://doi.org/10.2136/sssaj1978.03615995004200020010x
South DB, Harris SW, Barnett JP, Hainds MJ, Gjerstad DH (2005) Effect of container type and seedling size on survival and early height growth of Pinus palustris seedlings in Alabama, USA. For Ecol Manag 204:385–398. DOI: https://doi.org/10.1016/j.foreco.2004.09.016
Stanturf JA, Palik BJ, Dumroese RK (2014) Contemporary forest restoration: a review emphasizing function. For Ecol Manag 331:292–323. DOI: https://doi.org/10.1016/j.foreco.2014.07.029
Steffens B and Rasmussen A. 2016. The Physiology of Adventitious Roots. Plant Physiology 170:603–617
Stokes A, Atger C, Bengough AG, Fourcaud T, Sidle RC (2009) Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 324:1–30. DOI:https://doi.org/10.1007/s11104-009-0159-y
Sung S-JS, Haywood JD, Sword-Sayer MA, Connor KF, Scott AD (2010) Effects of container cavity size and copper coating on field performance of container-grown longleaf pine seedlings. In: Stanturf JA (ed) Proceedings of the 14th biennial southern silvicultural research conference. Gen Tech Rep SRS-GTR-121. USDA Forest Service, Southern Research Station, Asheville, NC, pp 241–245
Telewski FW, Moore JR (2016) Trait selection to improve wind firmness in trees. CAB Rev 11:1–10. DOI: https://doi.org/10.1079/PAVSNNR201611050
Thornley JHM (1972) A balanced quantitative model for root: shoot ratios in vegetative plants. Ann Bot 36:431–441. DOI: https://doi.org/10.1093/oxfordjournals.aob.a084602
Tsakaldimi MN, Ganatsas PP (2006) Effect of chemical root pruning on stem growth, root morphology and field performance of the Mediterranean pine Pinus halepensis Mill. Sci Hort 109:183–189. DOI: https://doi.org/10.1016/j.scienta.2006.04.007
Vogel JG, Jokela EJ (2011) Micronutrient limitations in two managed southern pine stands planted on Florida spodosols. Soil Sci Soc Am J 75:1117–1124. DOI: https://doi.org/10.2136/sssaj2010.0312
Wang FX, Wang ZY, Leeb JHW (2007) Acceleration of vegetation succession on eroded land by reforestation in a sub- tropical zone. Ecol Eng 31:232–241. DOI: https://doi.org/10.1016/j.ecoleng.2007.07.004
Wenny DL, Woollen RL (1989) Chemical root pruning improves the root system morphology of containerized seedlings. West J Appl For 4:15–17. DOI:https://doi.org/10.1093/wjaf/4.1.15
Wenny DL, Liu Y, Dumroese RK, Osborne HL (1988) First year field growth of chemically root pruned containerized seedlings. New For 2:111–118. DOI: https://doi.org/10.1007/BF00027762
Xu D, Miao J, Yumoto E, Yokota T, Asahina M, Watahiki M (2017) YUCCA9-mediated auxin biosynthesis and polar auxintransport synergistically regulate regeneration of root systems following root cutting. Plant Cell Physiol 58:1710–1723. DOI:https://doi.org/10.1093/pcp/pcx107
Yang M, Défossez P, Danjon F, Dupont S, Fourcaud T (2017) Which root architectural elements contribute the best to anchorage of Pinus species? Insights from in silico experiments. Plant Soil 411:275–291. DOI: https://doi.org/10.1007/s11104-016-2992-0
Zhao X, Zheng H, Li S, Yang C, Jiang J, Liu G (2014) The rooting of poplar cuttings: a review. New For 45:21–34. DOI: https://doi.org/10.1007/s11056-013-9389-1
Acknowledgements
This work is included in the activities of Task Force IUFRO “Transforming Forest Landscapes for future Climates and Human Well-being”. Authors are grateful to Dr. P.M. Chiarabaglio from CREA-Research Centre for Forestry and Wood (Casale Monferrato – AL, Italy) for providing poplar cutting material. Authors also thank Dr. Deborah S. Page-Dumroese, U.S. Department of Agriculture (USDA) Forest Service Rocky Mountain Research Station for a critical review of the manuscript. The authors are grateful to the reviewers and associate editor for their careful reading and valuable suggestions and comments that helped to improve the manuscript. The findings and conclusions in this publication are those of the authors and should not be construed to represent any official USDA or U.S. Government determination or policy.
Funding
This work was supported by the University of Insubria [FAR 2018–2020], and the EC FP7 [ZEPHYR, grant number 308313, 2012–2015]. Open access funding provided by Università degli Studi dell'Insubria within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Contributions
Conceptualization, A.M., R.K.D., and D.C.; methodology, A.M. and M.T.; software, M.T.; validation, A.M. and M.T.; formal analysis, A.M.; investigation, A.M., M.T. and G.N.; resources, A.M., G.S.S. and D.C.; data curation, M.T.; writing: original draft preparation, A.M.; writing: review and editing, A.M., R.K.D., and M.T.; visualization, M.T.; supervision, A.M., G.S.S. and D.C.; project administration, A.M.; funding acquisition, D.C. and A.M.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original paper has been corrected: Funding information has been updated.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montagnoli, A., Dumroese, R.K., Negri, G. et al. Asymmetrical copper root pruning may improve root traits for reforesting steep and/or windy sites. New Forests 53, 1093–1112 (2022). https://doi.org/10.1007/s11056-022-09913-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-022-09913-1