Abstract
We transplanted Shorea selanica and Shorea lamellata seedlings that either had or had not received ectomycorrhiza (EcM) Scleroderma columnare inoculum, commercially available and prescribed as standard practice in nursery, into rubber gardens of different age and plot history. The objective was to assess whether or not absence of fungal inoculants restricted seedling survival, growth, nutrient uptake and EcM formation in the first 2 years after out-planting in Jambi. Inoculation with EcM fungi in nursery had only limited positive effects on growth in height and diameter or N and P uptake, but it enhanced survival in the period 6–24 months after outplanting in all plots. With or without nursery stage inoculation, S. selanica and S. lamellata can be used for enrichment planting or reforestation in Sumatra as the species respond well to high light intensities. Presence of up to five morphotypes of EcM confirmed the availability of inoculum also in second generation rubber agroforests. Internal transcribe spacer sequencing revealed no S. columnare could be identified from the ectomycorrhizal roots of S. lamellata and S. selanica.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The natural vegetation in the lowlands of Western Indonesia consists of mixed dipterocarp rain forest (Whitten et al. 2000; Laumonier 1995). The Dipterocarpaceae is a pantropical family of 17 genera and approximately 500 species. It is well known for valuable timber trees traditionally used in residential construction, as well as for essential oils, balsam and resins (Soerianegera and Lemmens 1994). Under product names such as meranti, dipterocarps form the basis of Indonesia’s timber and plywood industry. In domesticated forests (Michon 2005), resin- (damar-) producing dipterocarps are of local importance in Southwest Sumatra (Torquebiau 1984).
In natural forests, dipterocarp trees form tall, cylindrical boles and characteristically dominate the canopy. The presence of wings on the seeds does not allow the large (11–59 mm diameter; Krishnapillay and Tompsett 1998) seeds to disperse far; dispersal for the most part is not more than 50 m from the mother tree (Osada et al. 2001). Seeds germinate directly, but this usually occurs in soil containing mycorrhizal roots close to the mother tree (Yasman 1995); the irregular seed production and difficulties of seed storage (absence of dormancy) are constraints to silvicultural use of the species. Most dipterocarps are shade tolerant and able to grow under limited light availability (Ashton 1998).
The trees have an ectomycorrhiza (EcM) association with fungi from either Basidiomycetes or Ascomycetes (Smits 1992, 1994; Lee et al. 1997; Wang and Qiu 2006). Among the Basidiomycetes, Russulaceae tend to dominate and can be studied by collecting their fruiting bodies (Smits 1994; Lee et al. 1997; Ingleby et al. 1998). Since the last decade molecular taxonomic techniques, such as polymerase chain reaction (PCR) and sequencing, has been used widely to identify EcM fungi from Basidiomycota and Ascomycota (Moyersoen 2006; Srikantaramas et al. 2003; Kovacs and Jakucs 2006; Tedersoo et al. 2006, 2007). Some dipterocarp species, such as Shorea balangeran, S. teysmanniana, and S. uliginosa, from peat swamp forest potentially have dual associations, in that they may form both ecto- and endomycorrhiza (Tawaraya et al. 2003).
Studies on the relationship between Dipterocarpaceae and EcM fungi in East Kalimantan showed that association with EcM fungi is necessary for early growth of dipterocarp seedlings, and that, in the nursery, inoculation may be necessary (Smits 1994; Yasman 1995; Priadjati 2002; Omon 2002). Direct contact with soil containing dipterocarp tree roots under ‘a mother tree’ in the nursery assists in the subsequent growth of dipterocarps used in enrichment planting of natural forest (Yasman 1995; Alexander et al. 1992). Survival of dipterocarp seedlings proved to have stronger dependence on EcM presence than on light intensity and soil properties (Yasman 1995). On the basis of these results, the Indonesian National Standard Agency has recommended using EcM inoculum in dipterocarp nurseries (Badan Standarisasi Nasional 2006). However, studies in Peninsular Malaysia showed less dependence on EcM inoculum (Lee and Lim 1989). We are not aware of critical studies in Sumatra.
After its introduction from Brazil at the end of the nineteenth century, the rubber tree Hevea brasiliensis became a major component of the local economy of the lowland forest zone in North Sumatra (Tengwall 1945), compatible with trees from the local flora in agroforest or ‘jungle rubber’ forms of land use (Gouyon et al. 1993; van Noordwijk et al. 1995; Joshi et al. 2003; Murdiyarso et al. 2002). The extensive management allows other trees to grow naturally from the seed bank and from newly dispersed seeds. The seedling and sapling diversity in rubber agroforests is as high as in natural forest, (Rasnovi 2006); but the tree composition differs from that of the natural forest (Beukema et al. 2007; Tata et al. 2008). The terminology of rubber garden, rubber agroforest (RAF) and species-rich RAF is based on basal area of rubber and that of other trees. In rubber gardens more than two-third of total basal area is formed by rubber; RAF has between one and two-thirds of its total basal area consisting of rubber trees; species-rich RAF has less than one-third of the total basal area formed by rubber, usually with at least five species of other trees.
Enrichment planting with valuable timber species in a rubber agroforest (RAF) context is an option that has yet to be explored. It has potential to meet the challenge of satisfying local demands for timber, but it is not yet widely practiced. RAF allows for a diverse floristic composition and creates the appropriate microclimate for late successional species, such as members of the Dipterocarpaceae. However, H. brasiliensis itself is not host of EcM fungi, nor are many of the trees that grow in RAF with it. This raises the concern that the effort to plant with dipterocarps may be constrained by the absence of EcM fungi in RAF soil. In this study Shorea selanica and S. lamellata seedlings were transplanted into rubber gardens of different age and condition, in order to:
-
Assess whether fungal inoculant increased the survival, growth, nutrient uptake and ectomycorrhiza formation of S. selanica and S. lamellata seedlings in the first 2 years after out-planting.
-
Assess the effect of RAF site history (time since conversion of rainforest to rubber agroforest) and condition on seedling survival, growth and nutrient uptake.
-
Identify constraints and other factors affecting growth of the two Shorea species tested in the RAF system.
Materials and methods
Study site
Five rubber gardens were studied. They differed in the age of the rubber trees and in the history of cultivation. Specifically, rubber gardens of 1, 5 and >10 years, derived directly from forest, and rubber gardens of 1 and 5 years, derived from mature RAF, were selected based on Landsat 7 ETM satellite imagery and on the interpretation of local land use changes in the past two decades. We used the ICRAF interpretation of satellite imagery for Jambi from 1973 to 2002 (Ekadinata and Vincent 2004) as well as later data from 2004. A remnant natural mixed dipterocarp forest (MDF) in Tebo district (belonging to the Silvagama education forest, Gadjahmada University), was used as a ‘forest control’ site. Geo-coordinates for each site were obtained using a Garmin GPS.
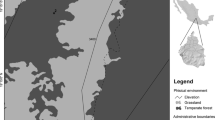
The Bungo and Tebo Districts are located in Jambi Province, Indonesia (101°52′–102°20′E and 1°30′–1°48′S, 50–250 m above sea level, a.s.l.), and lie on the undulating to flat basin areas of the Batang Bungo, Batang Pelepat and Batang Tebo rivers (Fig. 1), which are tributaries of the Batang Hari River. The area was selected as benchmark for the global ‘Alternatives to Slash and Burn’ program and details of land use and land use change are documented (van Noordwijk et al. 1995; Murdiyarso et al. 2002). The mean annual precipitation (in 2000 to 2006) in Tebo was 2,893 mm (cf. Sepunggur—ICRAF’s climate station), while in Bungo district it was 3,014 mm (cf. Muara Kuamang—ICRAF’s climate station) with a pronounced dry season from May to September. During the study period of 22 months, the total amount of precipitation in Sepunggur and Muara Kuamang was 3,678 mm (below long term average) and 5,341 mm (approximately long term average), respectively.
a Study area in the lowland of the Bungo and Tebo districts, Jambi Prov., Indonesia. b Land cover map based on satellite imagery of Landsat 7 ETM: (1) original mixed dipterocarp forest [forest]—2 plots; (2 and 6) rubber gardens 1–2 years old, derived from forest [ExFo_1]—1 plot each; (4 and 5) 5 year old rubber gardens derived from forest [ExFo_5]—1 plot each; (3) >10 year old rubber garden derived from forest [ExFo_10]—2 plots; (7) 1 year old rubber gardens derived from RAF [ExRAF_1]—2 plots; (8) 5 year old rubber gardens derived from RAF [ExRAF_5]—2 plots
The soils at the Bungo and Tebo sites are very deep, well drained, and very acid, and they have a low soil fertility status (available P Bray1 = 15.5; see below). Dominant soil types according to United States Department of Agriculture (USDA) soil classification are Oxisol, Kandiudox and Tropofluvent (van Noordwijk et al. 1995).
Seed germination
Seeds from two species of dipterocarps were collected in the arboretum of the Forest Research and Development Agency (FORDA), Bogor, Indonesia, in late September 2004. Shorea lamellata Foxw grows in undulating land and low hills and has medium size nuts (ca. 10–12 × 9–11 mm; Newman et al. 1996; Ashton 1982); its wood is classified in the timber trade as a light hardwood white meranti. Shorea selanica Bl grows naturally in lowland forest on well drained land with fertile soils and also has medium size nuts (ca. 15 × 8 mm; Ashton 1982); its wood belongs to the light red meranti group.
The seeds of S. lamellata and S. selanica were sown in sterilized mixed coco peat and rice husk (1:1) that had been autoclaved (121°C for 30 min). After the seeds had germinated (about 4 weeks after sowing), the seedlings (two leaved stage) of both Shorea species were transferred into a nursery at Babeko village, Bungo district, Jambi.
Media preparation and EcM fungi inoculation
The medium in which the seedlings were planted had been prepared prior to seedlings transfer. We used the top soil of rubber gardens mixed with rice husk (2:1). The mixed medium was sterilized chemically, using Basamid G (Dazomet 97%, Hoechst GmbH., Frankfurt, Germany) in a dosage of 100 g m−3 medium, in a closed plastic container. The medium was incubated for 2 weeks.
Seedlings were transferred into the sterilized medium in plastic bags of 1,000–1,200 cm3 each. A day after transplanting, a single 0.4 g tablet containing spores of the EcM fungus Scleroderma columnare, produced by the Laboratory of Forest Microbiology, Forest and Nature Conservation Research and Development Centre, Bogor, Indonesia (Turjaman et al. 2005), was inoculated into the potted seedling near the roots. Spore population of the tablet was 1.1 × 106 spore mg−1 and age of the spore was 6 months. Number of inoculated Shorea seedlings was 1,920. Control seedlings were not inoculated. The seedlings were watered regularly and maintained in the nursery for 3 months. In total 3,840 seedlings of both species were grown and distributed over the plots in the research sites with six different histories (see below).
Experimental design
Experimental plots were set up according to a split plot block randomized design in a factorial experiment (Gomez and Gomez 1984; Steel and Torrie 1960), with tree species as plots and inoculation levels as subplots. The histories of the six sites were:
-
1.
Forest: natural MDF, dominated by Dipterocarpaceae species, such as Dipterocarpus crinitus, Shorea bracteolata, S. palembanica, S. acuminata and S. gibbosa;
-
2.
ExFo_1: rubber age 1–2 years, site derived from forest;
-
3.
ExFo_5: rubber age 5 year, site derived from forest;
-
4.
ExFo_10: rubber age >10 year, site derived from forest;
-
5.
ExRAF_1: rubber age 1 year, site derived from previous RAF; at least 40 years since clearance from natural forest; and
-
6.
ExRAF_5: rubber age 5 years, site derived from previous RAF and about 50 years since clearance from natural forests.
At each site two experimental plots of about 1 ha each were selected to serve as replicates. Within each plot four quadrats of 240 m2 were established; the quadrats were at least 15 m apart. In each quadrat 80 seedlings were transplanted, either from S. lamellata or from S. selanica. In one of the quadrats of each species (randomly selected), seedlings inoculated in the nursery were used, while in the remaining plots uninoculated seedlings were used. The Shorea seedlings were planted between the rubber trees with planting space of 3 m × 5 m. Density of rubber trees varied from 500 to 650 rubber trees per hectare.
Plot management
Eight farmers from five different villages, viz. four farmers in the Bungo district (Babeko, Dusun Danau, Muara Kuamang and Koto Jayo villages); and four farmers in the Sei Srumpun village (Tebo district), collaborated in the study (Fig. 1). The farmers managed the plots according to their normal practice; areas around the planting holes were cleared at planting time in January 2005 and the planting rows were weeded twice a year after that.
Farmer’s management of the plots implied incomplete control of wild pigs and sheep and goat grazing. The intensity of animal disturbance, mainly due to wild pigs (Sus scrofa) on planted seedlings, was recorded on a single occasion, as was of the degree to which I. cylindrica had invaded the quadrats.
Field assessment
Light availability
Light availability in the study plots was measured using a Lux meter (Extech light meter) at midday on a single occasion in February 2007. Each subplot was measured once; therefore each block consisted of four measurements. It was expressed as the ratio of the light in the quadrat to the light in an open area (in %).
Survival and growth of planted seedlings
Height and stem diameter at 5 cm above ground level of the seedlings were recorded immediately at planting time. Survival and growth were monitored every 6 months for 2 years and the probability of survival over each measurement interval was estimated from the survival fractions.
Harvest
At 12 MAP, the height monitoring data were used to stratify the tree into three classes, viz small, medium, large; and trees were randomly selected for destructive sampling. The total of 522 seedlings, consisted of 264 seedlings of S. selanica and 258 seedlings of S. lamellata, were harvested by stratified random sampling (this amounted to ca. 20% of the seedlings surviving in each quadrat). The number of leaves was counted. Total biomass (including leaves, stems and roots) of seedlings was recorded after drying the plant samples at 70°C for 48 h. The concentrations of total N and P in the leaves was determined by means of the Kjeldhal and Bray1 methods, respectively, in the laboratory of Soil Chemistry, Centre for Soil and Agroclimate Research (CSAR) in Bogor, Indonesia.
The roots of each tree sample were traced from the stem base to be sure of their identities, within 0.5–1 m radius of the tree and the depth of 0–30 cm from top layer soils. The roots washed under tap water to separate them from soil. We randomly selected root tips from about 20% of the harvested seedlings and these were spread into Petri dishes. The remaining root biomass was dried weight. The number of root tips and roots with EcM were counted under a dissecting microscope and expressed as a fraction of the whole sample. Confirmation of EcM colonization was obtained by examining a cross section of the root tips using a compound microscope and recording the presence of a mantle and a Hartig net (Brundrett et al. 1996). The morphotype of the EcM was recorded according to Agerer (1987–1998). After morphotyping, up to 5 root segments of each morphotype, 1 cm in length, were taken from each root system and placed separately in 1.5 ml centrifuge tubes containing silica gel. They were labeled and stored in the freezer at −4°C. These samples were transferred to the Centraalbureau voor Schimmelcultures (CBS), Fungal Biodiversity Centre, Utrecht, the Netherlands for further analysis.
Soil analysis
Soil samples were collected randomly from the top soil (0–15 cm depth) in each quadrat and mixed to obtain a composite for every plots, i.e. 2 plots per site. In total, 12 soil samples were analyzed in the laboratory of Soil Chemistry, CSAR, in Bogor, Indonesia. The soil samples were analyzed for texture (sand, silt, clay), pH (in a 1:25 soil: solution extract with water or 1 N KCl), PBray1, Corg (Walkley and Black), Ntotal (Kjeldhal), exchangeable K, Ca, Mg, Na (exchanged with 1 N NH4-acetate solution pH 7) and exchangeable Al and H (exchanged with a 1 N KCl solution). The effective cation exchange capacity (ECEC) was obtained by summation of these cations. Initial soil conditions were assessed in May 2005. The reference organic C (Cref) content for forest soils was calculated using a regression equation derived from a large data set for Sumatra (van Noordwijk et al. 1997):
Molecular identification of EcM fungi
DNA extraction
DNA extraction from EcM root tips followed the protocol of in Möller et al. (1992) with modifications. The starting material for extraction consisted of 3–5 root segments of each morphotype from a single sampling code. This was transferred to a 2 ml screw-capped FastPrep tube containing glass beads (Sigma G9143) and 400 μl TE-extraction buffer (100 mM Tris, 40 mM Na-EDTA, pH adjusted to 9.0). Samples were homogenized for two times 3 min with TissueLyser (Qiagen Inc., Valencia, United State of America, USA). To this mixture, 120 μl of 10% sodium dedocyl sulfate SDS and 10 μl Proteinase K were added, and the tubes were vortexed. The mixture was incubated in a water bath at 55°C for 30 min. The mixture was then homogenized for two times 3 min with TissueLyser. The salt concentration was increased by adding 120 μl 5 M NaCl solution. The mixture was combined with 1/10 volume cetyltrimethylammonium bromide (CTAB) buffer 10%, followed by incubation at 55°C for 60 min. Material was homogenized with TissueLyser for two times 3 min. One volume of mixture solution of choloroform and isoamylalcohol (with ratio of 1:24, v/v) (SEVAG) was added and mixed gently by hand. After centrifugation at 14,000 rpm, 4°C, for 5 min, the top layer was transferred into a new and sterilized Eppendorf™ tube. NH4-acetate (225 μl 5 M) was added to the sample and mixed gently by inverting the tubes. After incubation for 30 min at 0°C, the mixture was centrifuged. The supernatant was transferred into a clean Eppendorf tube and 0.55 of volume ice-cold isopropanol (ca. 510 μl) was added. The mixture was incubated at −20°C for 60 min, followed by centrifugation at 4°C at 14,000 rpm for 5 min. The supernatant was decanted and the pellet was washed with 1 × 1,000 μl ice-cold 70% ethanol. The pellet was air-dried in a vacuum dryer (DNA 110 Speed Vac, GMI Inc., Minnesota, USA), for 10 min. The powder was resuspended in 100 μl Tris-EDTA buffer (pH 8.0). The DNA concentration was quantified with a NanoDrop™ 1000 spectrophotometer (Thermo Scientific, Wilmington, USA). The DNA samples were kept at −20°C until use.
DNA amplification
Polymerase chain reaction (PCR) was performed in 25 μl of a reaction mixture containing 2.5 μl 10× NH4 buffer, 2.5 μl dNTP Mix, 0.1 μl 5 U BioTaq™ (Bioline GmbH., Luckenwalde, Germany), 1 μl of 10 pmol forward and reverse primers, and 1 μl of 10–100 ng rDNA. Universal primer forward primer ITS1 (5′-TCCGTAGGTGAACCTGCGG-3′ (White et al. 1990) and a fungus-specific reverse primer NL6C (5′-CAAGTGTTTCCCTTTCAACA-3′; Egger 1995) were used. The amplifications were performed in a Gene Amp PCR system 9700 thermal cycler (Applied Biosystems, Foster City, CA) with the following parameters: initial denaturation at 94°C for 2 min, subsequently 30 cycles, consisting of denaturation at 94°C for 30 s, annealing at 52°C for 30 s, extension at 72°C for 70 s, with final extension at 72°C for 8 min. DNA bands were visualized with ethidium bromide and all samples were subjected to RFLP (restriction fragment length polymorphism) analysis.
RFLP analysis
RFLP analysis was conducted in order to select root-tip samples to be used for cloning and sequencing. PCR products were digested with restriction enzymes AluI, HhaI and HinfI (New England BioLabs Inc., Ipswich, MA). For each sample, 8 μl of PCR product was used with 9.4 μl sterile MilliQ water (Milipore Ltd., UK), 2 μl endonuclease buffer, 0.2 μl bovine serum albumine (New England BioLabs Inc., Ipswich, MA) and the three restriction enzymes to final concentrations of 10 U μl−1 each. Fragments were separated on 2% Ultrapure™ agarose (Invitrogen Ltd., Paisley, UK) in 1× TAE (Tris-acetate-EDTA, 40 mM Tris-acetate and 1 mM EDTA, pH 8.5) buffer, at 100 V for 15 min and subsequently at 150 V for 3 h.
Cloning and sequencing
In case where amplicons were similar in size after electrophoresis, further separation was accomplished by cloning using TOPO TA® kits (Invitrogen, Paisley, United Kingdom, UK). One sample was cloned to 20 bacterial colonies on Luria Bertani Agar (Sigma Aldrich®, USA) amended with 50 μg ml−1 ampicillin (Fluka 10044), 2% X-gal (Promega V3941) and 0.1 M IPTG (Isopropyl-d-thiogalactoside; Sigma I-6758). Cloning followed the protocol available from the kit. PCR was then performed in 10–20 bacterial colonies from each ligation using primers M13 forward (5′-GTAAAACGACGGCCAGT-3′) and M13 reverse (5′-GGAAACAGCTATGAC-3′). Sequencing PCR used 1 μl of template DNA (1–10 ng) with reaction mix solution mentioned above; other conditions were as follows: initial denaturation at 94°C for 3 min, then 28 cycles of denaturation at 93°C for 1 min, annealing at 52°C for 1 min, extension at 72°C for 2 min, with final extension at 72°C for 3 min, and cooling down to 8°C.
The PCR products were sequenced using 1 μl template DNA (1–10 ng), 3 μl dilution buffer, 1 μl BigDye® Terminator (Applied Biosystems, Foster City, CA, USA) and 1 μl of 4 pmol primer in a 10 μl total volume with ultrapure water, as follows: initial denaturation at 95°C for 2 min, then 30 cycles of denaturation at 95°C for 10 s, annealing at 50°C for 5 s, extension at 60°C for 2 min, cooling down to 8°C. Sequencing products were purified with Sephadex G-50 Fine (GE Healthcare, Uppsala, Sweden) and analyzed by using an ABI Prism 3730 DNA analyzer (Applied Biosystems).
Molecular identification
The sequences were adjusted by using software programme SeqMan II (DNAStar Inc.). The identity of the sequences, i.e. nearest neighbors, was determined using GenBank (Altschul et al. 1997; Zhang et al. 2000) and some dedicated databases at the CBS.
Data analysis
Basic statistical analyses were conducted using GenStat 9th Edition for windows (VSN International Ltd., U.K.). Data were checked for homogeneity of variance and normality by analysis of the residual. Data on survival, growth in height and stem diameter and number of leaves were log10 transformed, while EcM colonization was log10 transformed after adding 1 unit. Repeated measure procedure was applied for data on survival, growth in height and stem diameter. The N and P concentrations of the shoots were subjected to analysis of using the GLM procedure of Statistica ver.6 (StatSoft Inc., U.S.A.).
Absolute growth rates (AGR) of height and stem diameter were calculated as the increment between two consecutive measurements 6 month apart. The relative growth rate of stem diameters (RGRD) was calculated as:
where D 1 and D 2 are stem diameters measured at times 1 and 2, which were 6 months apart.
The relative height growth rate (RGRH) was calculated as:
where H 1 and H 2 are height at times 1 and 2, again 6 months apart.
Four specific contrasts (Table 1) where tested in the ANOVA within the 5 degrees of freedom of the ‘land history/condition’ factor: (1) reflecting the time (on a logarithmic scale, scaled back to achieve a mean of zero), (2) reflecting the current light level, (3) the primary texture-related soil variables and (4) the first (ex Forest) or second (ex RAF) agroforest cycle.
Occurrence of animal disturbance and I. cylindrica were used as covariates for tree response parameters. Disturbance by animals was ranked into two numerical classes: 0 (no disturbance), or 1 (disturbed). Presence of I. cylindrica was ranked as 0 (none), 1 (rare) or 2 (abundant).
Results
Light availability and soil properties
Light availability varied among the six study sites. The lowest light intensity occurred in the forest (2.7%), while the highest was found in site ExFo_1, forest-derived plots with 1 year old rubber seedlings (49.4%).
All sites had very acid soils of low nutrient content, with the highest pH (as measured in KCl extracts) of 4.1 recorded for site ExFo_1—plot #2) and the lowest value of 3.5 for the forest. The textures ranged from sandy clay loam to clay. Sand content generally was high (49–71%), except in both plots of site type ExRAF_1, where values ranged from 16 to 22%.
Table 2 analyzes the soil properties for the three contrasts, to test for possible confounding factors in subsequent interpretation of tree growth. The relative soil C content (C/Cref) was close to 1.0 in the forest at 0.92 ± 0.01 and was lower in RAF plots derived from forest 0.66 ± 0.04 or in second-generation RAF plots 0.63 ± 0.10. The TimeSinceConversion contrasts were associated, as expected, with a decline in the C/Cref ratio (but not with organic C without correction for texture via the reference value) and with an increase in pH (as measured in KCl extracts) and an associated decrease in exchangeable Al3+. They were also associated with a decrease in the fraction of the soil made up by silt and ECEC/Clay.
The contrast CurrentLightLevel was associated with decrease in silt and ECEC/clay and with an increase in pH (in KCl extracts) but not with a decrease in exchangeable Al3+ or with relative Al saturation. The soil texture contrast termed the ‘sand minus clay’ factor was negatively associated with the organic C and N content of the soil as well as with available P and exchangeable Mg2+ and K+. It was not associated with C/Cref or soil pH. The total Fe content and the exchangeable Ca2+ content in the soil varied considerably among sites (0.9–5.8%, and 0.2–2.6 cmol+kg−1, respectively) but neither factor was associated with any of the three contrasts. The forest and forest-derived plots had high Al saturation (mean 72%). Second generation rubber agroforest plots tended toward lower aluminum saturation (Fig. 2).
a Relationship between pH and Al saturation in the top soil of the six plots types; b Relationship between the ‘sand minus clay’ factor and available P at all sites. ExFo_1 year old rubber trees, plot derived from forest; ExFo_5: 5 year old rubber trees, plot derived from forest; ExFo_10: >10 year old rubber trees, plot derived from forest; ExRAF_1: 1 year old rubber trees, plot derived from RAF; ExRAF_5: 5 year old rubber trees, plot derived from RAF
Early growth in nursery
EcM inoculation affected early growth of height (F {1,3809} = 7.8; P = 0.005) and stem diameter (F {1,1143} = 43.3; P = 0.0001) of the two Shorea species at age 3 months in nursery before planting out in the field (Table 3). Visual inspection of seedlings before planting out showed that nearly half of inoculated seedlings to have EcM roots. Non-inoculated seedlings did have EcM roots but less. No specific identities of EcM fungi colonized the seedlings could be established, however.
Survival rate in the field
Mortality was high between 6 and 18 months for both S. selanica and S. lamellata. During 2 years of observation, the survival rate of both seedlings inoculated with EcM was higher than that of the uninoculated seedlings, except for S. lamellata planted in ExRAF_5. After 2 years, the highest survival rate was seen in inoculated S. lamellata and S. selanica planted in ExFo_10, while the lowest survival rate was seen with the uninoculated S. selanica and the inoculated S. lamellata planted in ExRAF_5.
The ANOVA (Table 4) and means (Table 5) showed that the Shorea species did not affect the survival rate, while EcM inoculation yielded a significant effect only at the third period of measurement. The history/condition of the plots affected the survival of the two Shorea species. The interaction of EcM inoculation (E), history/condition of land (L) and species (T) was significant only at the fourth period of measurement.
Relative growth rate of height (RGRH) and stem diameter (RGRD)
Tree growth varied primarily between sites (L), with few differences between the tree species (T) and effects of inoculation [E] (Tables 4, 5). Contrasts L1 and L2 contributed most to the L effect and the ex-Forest versus ex-RAF contrast was not statistically significant. The longer the time since forest clearing and the higher the light availability, the higher RGRH and RGRD in period 2 (1 year after planting). Interaction between land history and tree species was statistically significant only in 2 out of 16 comparisons. No statistically significant effect was noted for interaction between land history and the impact of EcM inoculation on tree growth.
A statistically significant interaction between EcM inoculation and land history/condition was found at the first measurement of RGRH and the last of RGRD, but the trend seen was not in the direction hypothesized: the positive inoculation effect was smallest rather than largest in the plots with the longest history of cultivation since forest clearing. The three-way interaction of species, EcM inoculation and land history/condition was significant for RGRH only at the second period of measurement.
S:R (shoot:root) ratio, number of leaves, and biomass
Seedlings planted at site ExRAF_1 had the highest number of leaves and biomass. After 1 year, the S:R ratio, the dry weight of leaves and roots and EcM colonization were affected by EcM inoculation, while history of land or associated light levels significantly affected number of leaves and biomass, e.g. dry weight (DW) of leaves, stems and roots. No interaction effect was shown for all response variables. The effect of EcM inoculation on the S:R ratio varied among sites and between species. EcM inoculation did not always increase the S:R ratio. Inoculation with EcM increased the number of leaves and biomass (DW of leaves, stems and roots) of S. lamellata and S. selanica planted at all sites, except for S. lamellata at ExRAF_5.
Concentration of nitrogen and phosphorus
ANOVA results showed that history/condition of sites and the interaction between site history/condition and EcM inoculation significantly effected shoot nutrient (N and P) concentration. EcM inoculation did not affect shoot N and P concentration of the two Shorea species. Seedlings in ExRAF_5 had the lowest N concentration while those in ExForest_10 had the lowest P concentration (Table 6).
EcM colonization
The effect of EcM inoculation on EcM colonization varied among treatments. EcM colonization of inoculated seedlings was not always higher than uninoculated seedlings. At the age of 12 MAP, uninoculated S. selanica planted at forest had no EcM colonization, while uninoculated S. selanica planted at ExRAF_5 had high EcM colonization. EcM colonization of uninoculated S. lamellata planted at forest showed moderate EcM colonization in the forest plot. We found five EcM morphotypes on S. selanica and S. lamellata roots. Morphotype T1, featuring simple monopodial to regularly pinnate branching and brownish mantle colors, was very common in seedlings planted at forest, ExForest_1, ExForest_5, ExForest_10 and ExRAF_5 sites. Morphotype T5, showing irregular clumpy branches, black mantle color, and black emanating hyphae, was very common in seedlings planted at ExRAF_1. The frequency of each morphotype in relation to the land history is shown in Table 7. Inoculation did not lead to a systematic increase of any of the EcM morphotypes.
EcM fungal identity
Two hundred and seven DNA samples from EcM root tips collected in the study area in Jambi yielded 19 distinct RFLP patterns. The cloned PCR products yielded 1–5 distinct sequences from each ligation. A total of 77% of the clones could be identified as EcM fungi by molecular identification of EcM root tips based on general taxonomic affinities of GenBank sequences (Fig. 3). Molecular identification was possible for all 19 RFLP patterns. Tomentella was the most common genus, consisting of 6 (distinct but unnamed) species, followed by Pisolithus (3 species), Clavulina, Sebacina and Sistotrema (1 species each; Table 8).
Fungi indentified on morphotype T1 all corresponded with Tomentella, T2 Tomentella or Pisolithus, T3 Tomentella, Clavulina or Sistotrema and T4 Tomentella (or other Telephoraceae) or Sebacina. No identification was successful on morphotype T5.
Discussion
The limited response of survival and growth to inoculation and the abundance of EcM of different morphotypes in all locations where S. lamellata and S. selanica were outplanted was unexpected and different from what has been reported for early growth of dipterocarp species from Borneo (Smits 1994; Turjaman et al. 2007; Brearley et al. 2007). Our results challenge, at least for lowland Sumatra, the concept that lack of EcM is a primary constraint on the use of dipterocarps for enrichment planting. Data from Peninsular Malaysia however, in fact also showed little response of EcM inoculation on two species of Dipterocarpaceae (Chang et al. 1996) and even no positive response to enhance tree establishment, growth and survival (Lee et al. 1996; Lee 2006).
Land history and light condition effects
Our results indicated that early survival of S. lamellata and S. selanica was influenced by land history more than by EcM inoculation. The ‘current light conditions’ factor had more impact on survival and growth than did ‘land history since forest conversion’. The two Shorea species grew very slowly in the forest plot, but they did manifest a positive response to inoculation in this environment. The canopy of forest was dense, and light availability was low; these conditions may be unsuitable for the growth of the test species. Seedlings of both species grew better in the open areas, such as ExForest_1 and ExRAF_1. Previous studies have shown growth of dipterocarp seedlings (mainly Shorea) to be enhanced in well illuminated conditions (Tennakoon et al. 2005; Brearley et al. 2007) and to show a positive correlation with gap distance (Otsamo 2000).
Regarding EcM colonization, land history did not have a significant effect. Both Shorea species planted in the relatively exposed conditions of RAF generally showed higher EcM colonization than those planted in the forest site. Previous reports have shown better mycorrhization for several species of dipterocarp seedlings when they are planted under high light conditions in an open canopy rather than under closed canopy (Ingleby et al. 1998; Tennakoon et al. 2005; Brearley et al. 2007). Gehring (2004) found a similar effect of light intensity on mycorrhization of Chrysophyllum (Apocynaceae) seedlings. This suggests that higher levels of photosynthesis in more open areas support mycorrhization while a dense forest canopy limits carbohydrate availability in tree seedlings and thus also limits EcM formation and function (Ingleby et al. 1998; Bucking and Heyser 2001).
High light availability in RAFs did not decrease EcM colonization or the number of EcM types. Ingleby et al. (1998) similarly found that under a relatively open canopy, S. parvifolia seedlings had higher EcM colonization and more diverse EcM than those found under a closed canopy. They found that many EcM types present in a more open canopy were not encountered on seedlings under a closed canopy. Our result showed that the mycobiont of morphotype T5 (e.g. ramification irregular pinnate and coralloid; mycorrhizae and emanating hyphae black) appeared to be well adapted to growth on seedlings experiencing high light availability in more open areas. A high frequency of morphotype T5 was observed in ExRAF_1 and ExForest_1, but not in forest. We suspect that the mycobiont of morphotype T5 occurs indigenously in the soils of Bungo and Tebo districts.
EcM inoculation effect
Inoculation of Scleroderma columnare accelerated early growth of height and diameter on S. lamellata and S. selanica for 3 months in the nursery before they out planted to the field. It supported many other reports on the benefit of EcM inoculation to dipterocarp seedlings (Turjaman et al. 2005; Tennakoon et al. 2005). After out planted to the field, however, positive effects of EcM inoculation in the nursery were observed mainly at the end of the first year. Beyond that point, EcM inoculation had no effect on the growth and survival of either test species. Similar results for endomycorrhizal inoculation were reported by Murniati (2002), who found positive effects of nursery-stage inoculation on initial survival but then detected no influence on subsequent growth of four endomycorrhizally associated tree species planted in Imperata grassland in East Kalimantan. Mycorrhiza formation does not always increase plant fitness (e.g. survival and growth), but this depends on the specific plant, fungal genotypes and abiotic and biotic environments (Jones and Smith 2004), and quality of the natural inoculum in the nursery (Le Tacon et al. 1992).
In contrast to findings of Turjaman et al. (2007), inoculation of dipterocarps with EcM in the nursery stage did not increase shoot N and P tissue concentration or total uptake in our experiment. Jones and Smith (2004) suggested that transfer of P from the fungus to the plant does not necessarily mean that the plant will accumulate more P or is able to grow faster, because the fungus gains a high proportion of the P, while there is no overall increase of P in the plant. Site ExRAF_1, which had the highest available P content in the soils, yielded the highest P concentration in the foliar for both uninoculated and inoculated seedlings. In any event, phosphorus relations may have little influence in general on the degree of mycorrhization. Indeed, the fact that addition of phosphate fertilizer does not reduce EcM colonization (Brearley et al. 2007; Baxter and Dighton 2005) and EcM richness (Baxter and Dighton 2005) has been cited as evidence that the EcM symbiosis is an obligate relationship, i.e., one not abolished by ideal nutrient conditions and therefore one not necessarily stimulated by nutrient deprivation.
After 1 year out planted in the field, EcM colonization of uninoculated S. lamellata at all sites and uninoculated S. selanica at some sites was relatively high compared with inoculated seedlings. Direct sequencing from EcM root tips showed that no Scleroderma columnare was identified from the EcM root tips samples from inoculated or non-inoculated Shorea seedlings.
EcM fungal identity
Molecular techniques, i.e. ITS-PCR and direct sequencing applied in this research, could identify the EcM fungal colonizers of root tips of S. lamellata and S. selanica out-planted in the field. The diversity of morphotypes on seedling planted in RAF sites in our study appears to reflect the abundance of indigenous mycorrhizal inoculum. A total of 11 taxa of EcM fungi could be identified based on ITS-sequence, but this may not represent the total diversity. No Scleroderma columnare fungus was positively identified from either inoculated or uninoculated seedlings. This implies that the nursery-type EcM (assuming it was the EcM type seen on the inoculated seedlings) was replaced by native EcM fungi within a year. Unfortunately, we did not have DNA confirmation of Scleroderma columnare from the seedlings at the time before out planted in the field.
Although data of fungal identity from the seedlings planted out in the forest are not available, the seedlings planted 10 years after forest conversion yielded a greater diversity of EcM types than plots after a RAF cycle (Table 8). The inoculants can have survived in the soil, or reached the seedlings from the neighboring landscape, such as remnant forests and mature RAFs, where EcM host trees are still present. The closest plot to the remnant forest patch was about 2 km, (plot ExForest_1), and the closest plot to species rich RAF was about 1 km (plot ExRAF_1). Fungal inoculum may be dispersed by airborne dispersal, fungivores and sporivores. Mites (Oribatida), beetles (Coleoptera), flies (Diptera) and springtails (Collembola) may act as dispersal agent (Lilleskov and Bruns 2005). Large mammals, like wild pigs (Sus scrofa), which are common in the surroundings of the study area in Jambi, may also play a role. An indication may be the finding of fungal fragments in fecal materials of Sus barbatus (Setyowati et al. 2005; Meijaard et al. 2005). Such agents may allow colonization of S. lamellata and S. selanica planted in the RAF. Further studies of fungal ecology may be needed to clarify the pathways of survival and time frame for survival of inoculum.
Rubber normally has arbuscular mycorrhiza (AM) (Ikram et al. 1992; Schwob et al. 1999). Some Dipterocarpaceae have been reported with dual colonization of EcM and AM fungi (Tawaraya et al. 2003). Our molecular identification, however, showed no DNA sample similar to the AM fungus sequence in the Genbank, so dual colonization is unlikely for our Shorea species tested.
Plot management
In the establishment of smallholder rubber plantings in Jambi, the most significant practical problem noted was damage by vertebrate pests, such as wild pigs, (Sus scrofa), monkeys (Presbytis melalophos nobilis) and sheep (Ovis aries) (Williams et al. 2001). Wild pigs damaged Shorea planted in the RAF sites but there was no evidence of damage in MDF. When we used animal disturbance and presence of I. cylindrica as covariates, it did not affect the results.
We conclude that in lowland Sumatra or at least in Jambi, RAF provides suitable sites for enrichment planting with dipterocarp trees. Inoculation of EcM inoculum in the nursery had positive effect on the early growth of height and stem diameter. After seedlings have been out planted to the field, EcM inoculation provides a small increase in seedling survival rate but is not essential, since EcM inoculum potential persists in the soil after natural forest was changed to RAF. S. selanica and S. lamellata can be selected for use in enrichment planting, afforestation and reforestation in Sumatra under conditions where there is a partially open canopy.
References
Agerer R (1987–1998) Colour atlas of ectomycorrhiza. Einhorn-Verlag Eduard Dietenberger, Munchen
Alexander I, Ahmad N, Lee SS (1992) The role of mycorrhizas in the regeneration of some Malaysian forest trees. Phil Trans R Soc B 335:379–388. doi:10.1098/rstb.1992.0029
Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402. doi:10.1093/nar/25.17.3389
Ashton PS (1982) Dipterocarpaceae. In: Van Steenis CGGJ (ed). Flora Malesiana 1(9):237–552
Ashton MS (1998) Seedling ecology of the mixed-dipterocarp forest. In: Appanah S, Turnbull JM (eds) A review of dipterocarps: taxonomy, ecology and silviculture. CIFOR and FRIM. Center for International Forestry Research, Bogor, pp 133–150
Badan Standarisasi Nasional (2006) Inokulasi cendawan ektomikoriza pada bibit tanaman kehutanan. SNI 01-7225-2006. Jakarta. URL: http://www.bsn.or.id/files/sni/SNI%2001-7225-2006.pdf. Accessed 11 Aug 2008
Baxter JW, Dighton J (2005) Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza 15:513–523. doi:10.1007/s00572-005-0359-0
Beukema H, Danielsen F, Vincent G, Hardiwinoto S, van Andel J (2007) Plant and bird diversity in rubber agroforests in the lowlands of Sumatra, Indonesia. Agrofor Syst 70:217–242. doi:10.1007/s10457-007-9037-x
Brearley FQ, Scholes JD, Press MC, Götz Palfner G (2007) How does light and phosphorus fertilisation affect the growth and ectomycorrhizal community of two contrasting dipterocarp species? Plant Ecol 192:237–249. doi:10.1007/s11258-007-9325-6
Brundrett M, Bougher N, Dell B, Grove T, Malajczuk N (1996) Working with mycorrhizas in forestry and agriculture. Australian Centre for International Agricultural Research, Canberra
Bűcking H, Heyser W (2001) Microautoradiographic localization of phosphate and carbohydrates in mycorrhizal roots of Populus tremula × Populus alba and the implications for transfer processes in ectomycorrhizal associations. Tree Physiol 21:101–107
Chang YS, Lapeyrie F, Lee SS (1996) The survival and competitivenss of Pisolithus tinctorius on outplanted seedlings of Dipterocarpus alatus and Shorea glauca in Malaysia: preliminary report. In: Appanah S, Khoo KC (eds) Proceedings of the fifth round table conference on dipterocarps, Chiang Mai, Thailand. Forest Research Institute Malaysia, Kepong, 7–9 November 1994, pp 165–169
Egger KN (1995) Molecular analysis of ectomycorrhizal fungal communities. Can J Bot 73(S1):1415–1422. doi:10.1139/b95-405
Ekadinata A, Vincent G (2004) Assessing landcover dynamics in Bungo district, Jambi using multitemporal satellite imagery. SAU working document No. 12 (ICRAF internal report)
Gehring CA (2004) Seed reserves and light intensity affect the growth and mycorrhiza development of the seedlings of an Australian rain-forest tree. J Trop Ecol 20:345–349. doi:10.1017/S0266467403001160
Gomez KA, Gomez AA (1984) Statistical procedures for agriculture research, 2nd edn. Wiley, New York
Gouyon A, de Foresta H, Levang P (1993) Does ‘Jungle Rubber’ deserve its name? An analysis of rubber agroforestry system in Southeast Asia. Agrofor Syst 22:181–206. doi:10.1007/BF00705233
Ikram A, Mahmud AW, Ghani MN, Ibrahim MT, Zainal AB (1992) Field nursery inoculation of Hevea brasiliensis Muell. Arg. Seedling rootstock with vesicular-arbuscular mycorrhizal (VAM) fungi. Plant Soil 145:231–236. doi:10.1007/BF00010351
Ingleby K, Munro RC, Noor M, Mason PA, Clearwater MJ (1998) Ectomycorrhizal populations and growth of Shorea parvifolia (Dipterocarpaceae) seedlings regenerating under three different forest canopies following logging. For Ecol Manage 111:171–179. doi:10.1016/S0378-1127(98)00324-7
Jones MD, Smith SE (2004) Exploring functional definitions of mycorrhizas: are mycorrhizas always mutualisms? Can J Bot 82:1089–1109. doi:10.1139/b04-110
Joshi L, Wibawa G, Beukema HJ, Williams SE, Van Noordwijk M (2003) Technological change and biodiversity in the rubber agroecosystem. In: Vandermeer JH (ed) Tropical agroecosystems: new directions for research. CRC Press, Boca Raton, pp 133–157
Kovacs GM, Jakucs E (2006) Morphological and molecular comparison of white truffle ectomycorrhizae. Mycorrhiza 16:567–574. doi:10.1007/s00572-006-0071-8
Krishnapillay B, Tompsett PB (1998) Seed handling. In: Appanah S, Turnbull JM (eds) A review of dipterocarps: taxonomy, ecology and silviculture (chap 4). CIFOR, Bogor, pp 3–88
Laumonier Y (1995) The vegetation and physiography of Sumatra. Geobotany 22. Kluwerr, Dordrecht
Le Tacon F, Alvarez IF, Bouchard D, Henrion B, Jackson RM, Luff S, Parlade JI, Para J, Stenstrom E, Villeneuve N, Walker C (1992) Variations in field response of forest trees to nursery ectomyocchizal inoculation in Europe. In: Read DJ, Lewis DJ, Fitter AH, Alexander IJ (eds) Mycorrhiza in ecosystems. CAB International, Wallingford, pp 119–134
Lee SS (2006) Mycorrhizal research in Malaysian plantation. In: Suzuki K, Ishii K, Sakurai S, Sasaki S (eds) Plantation technology in tropical forest science. Springer, Tokyo, pp 157–166
Lee SS, Lim KL (1989) Mycorrhizal infection and foliar phosphorus content of seedlings of three dipterocarp species growing in a selectively logged forest and a forest plantation. Plant Soil 117:237–241. doi:10.1007/BF02220717
Lee SS, Alexander IJ, Moura-Costa P, Yap SW (1996) Mycorrhizal infection of dipterocarp seedlings in logged and undisturbed forest. In: Appanah S, Khoo KC (eds) Proceedings of the fifth round table conference on dipterocarps, Chiang Mai, Thailand. Forest Research Institute Malaysia, Kepong, 7–9 Nov 1994, pp 157–164
Lee SS, Alexander IJ, Watling R (1997) Ectomycorrhizas and putative ectomycorrhizal fungi of Shorea leprosula Miq (Dipterocarpaceae). Mycorrhiza 7:63–81. doi:10.1007/s005720050165
Lilleskov EA, Bruns TD (2005) Spore dispersal of a resupinate ectomycorrhizal fungus, Tomentella sublilacina, via soil food webs. Mycologia 97:762–769. doi:10.3852/mycologia.97.4.762
Meijaard E, Sheil D, Nasi R, Augeri D, Rosenbaum B, Iskandar D, Setyawati T, Lammertink M, Rachmatika I, Wong A, Soehartono T, Stanley S, O’Brien T (2005) Life after logging: reconciling wildlife conservation and production forestry in Indonesia Borneo. Center for International Forestry Research (CIFOR), Bogor
Michon G (2005) Domesticating forests: how farmers manage forest resources. IRD, CIFOR and ICRAF, Bogor
Möller EM, Bahnweg G, Sandermann H, Geiger HH (1992) A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20:6115–6116. doi:10.1093/nar/20.22.6115
Moyersoen B (2006) Pakaraimaea dipterocapacea is ectomycorrhizal, indicating an ancient Gondwanaland origin for the ectomycorrhizal habit in Dipterocarpaceae. New Phytol 172:753–762. doi:10.1111/j.1469-8137.2006.01860.x
Murdiyarso D, Van Noordwijk M, Wasrin UR, Tomich TP, Gillison AN (2002) Environmental benefits and sustainable land-use options in the Jambi transect, Sumatra, Indonesia. J Veg Sci 13:429–438
Murniati (2002) From Imperata cylindrica grasslands to productive agroforestry. Ph.D. thesis, Wageningen University. Tropenbos Kalimantan series 9. Tropenbos International, Wageningen, The Netherlands
Newman MF, Burgess PF, Whitmore TC (1996). Sumatra light hardwoods: Anisoptera, Parashorea, Shorea (red, white and yellow meranti). Manual of dipterocarps for foresters. Royal Botanic Garden, Edinburgh, U.K. Center for International Forestry Research. Bogor, Indonesia
Omon RM (2002) Dipterocarpaceae: Shorea leprosula Miq. Cuttings, mycorrhizae and nutrients. Ph.D. thesis, Wageningen University. Tropenbos-Kalimantan series 7. MoF-Tropenbos-Kalimantan Project, Indonesia
Osada N, Takeda H, Furukawa A, Awang M (2001) Fruit dispersal of two dipterocarp species in Malaysian rain forest. J Trop Ecol 17:911–917. doi:10.1017/S0266467401001687
Otsamo R (2000) Early development of three planted indigenous tree species and natural understorey vegetation in artificial gaps in an Acacia mangium stand on an Imperata cylindrica grassland site in South Kalimantan, Indonesia. New For 19:51–68. doi:10.1023/A:1006685103365
Priadjati A (2002) Dipterocarpaceae: forest fire and forest recovery. Ph.D. thesis, Wageningen University. Tropenbos-Kalimantan series 8. Tropenbos International. Wageningen, The Netherlands
Rasnovi S (2006) Ekologi regenerasi tumbuhan berkayu pada sistem agroforest karet. Sekolah Pasca Sarjana, Institut Pertanian Bogor. Bogor, Indonesia (unpublished dissertation in Bahasa Indonesia, with English abstract)
Schwob I, Ducher M, Coudret A (1999) Effect of climatic factors on native arbuscular mycorrhizae and Meloidogyne exigua in a Brazilian rubber tree (Hevea brasiliensis) plantation. Plant Pathol 48:19–25. doi:10.1046/j.1365-3059.1999.00300.x
Setyowati T, Read S, Coulson G, Sheil D (2005) Stomach and fecal analysis of the beared pigs (Sus barbatus) in lowland dipterocarp of East Kalimantan, Indonesia. Poster presentation in the IUFRO conference, Brisbane, Australia
Smits WTM (1992) Mycorrhizal studies in dipterocarp forests in Indonesia. In: Read DJ, Lewis DH, Fitter AH, Alexander IJ (eds) Mycorrhizas in ecosystems. CAB International, UK, pp 283–292
Smits W (1994) Dipterocarpaceae: mycorrhizae and regeneration. Tropenbos series 9. The Tropenbos Foundation, Wageningen
Soerianegera I, Lemmens RHMJ (1994) Major commercial timbers 5(1). Prosea foundation, Bogor, Indonesia
Srikantaramas S, Sugioka N, Lee SS, Ibrahim FH, Lee HS, Szmidt AE, Yamazaki T (2003) Molecular identification of ectomycorrhizal fungi associated with Dipterocarpaceae. Tropics 13:69–77. doi:10.3759/tropics.13.69
Steel RGD, Torrie JH (1960) Principles and procedures of statistics. McGraw, New York
Tata HL, van Noordwijk M, Werger M (2008) Trees and regeneration in rubber agroforests and other forest-derived vegetation in Jambi (Sumatra, Indonesia). JFR 5(1):1–20. http://www.forda-mof.org/uploads/File/JFR/V5-1-2008.pdf
Tawaraya K, Takaya Y, Turjaman M, Tuah SJ, Limin SH, Tamai Y, Cha JY, Wagatsuma T, Osaki M (2003) Arbuscular mycorrhizal colonization of tree species grown in peat swamp forests of Central Kalimantan, Indonesia. For Ecol Manage 182:381–386. doi:10.1016/S0378-1127(03)00086-0
Tedersoo L, Hanse K, Perry BA, Kjøller R (2006) Molecular and morphological diversity of pezizalean ectomycorrhiza. New Phytol 170:581–596. doi:10.1111/j.1469-8137.2006.01678.x
Tedersoo L, Suvi T, Beaver K, Kõljalg U (2007) Ectomycorrhizal fungi of the Seycheles: diversity patterns and host shifts from the native Vateriopsis seychellarum (Dipterocarpaceae) and Instia bijuga (Caesalpinaceae) to the introduced Eucalysptus robusta (Myrtaceae), but not Pinus caribea (Pinaceae). New Phytol 175:321–333. doi:10.1111/j.1469-8137.2007.02104.x
Tengwall TA (1945) The history of rubber cultivation and research in Netherlands Indies. In: Honig P, Verdroon F (eds) Science and scientist in the Netherlands Indies. New York City, Board for the Netherlands Indies, Surinam and Curacao, pp 344–351. http://www.knaw.nl/indonesia/Honig_verdoorn/Honig46.pdf
Tennakoon MMD, Gunatilleke IAUN, Hafeel KM, Seneviratne G, Gunatilleke CVS, Ashton PMS (2005) Ectomychorrhizal colonization and seedling growth of Shorea (Dipterocarpaceae) species in simulated shade environments of a Sri Lankan rain forest. For Ecol Manage 208:399–405. doi:10.1016/j.foreco.2004.12.010
Torquebiau E (1984) Man-made dipterocarp forest in Sumatra. Agrofor Syst 2:103–127
Turjaman M, Tamai Y, Segah H, Limin SH, Cha JY, Osaki M, Tawaraya K (2005) Inoculation with the ectomycorrhizal fungi Pisolithus arhizus and Scleroderma sp. improves early growth of Shorea pinanga nursery seedlings. New For 30:67–73. doi:10.1007/s11056-004-1954-1
Turjaman M, Saito M, Santoso E, Susanto A, Gaman S, Limin SH, Shibuya M, Takahashi K, Tamai Y, Osaki M, Tawaraya K (2007) Effect of ectomycorrhizal fungi inoculated on Shorea balangeran under field conditions in peat-swamp forests. In: Rieley JO, Banks CJ, Radjagukguk B (eds) Carbon-climate-human interaction on tropical Peatland. Proceedings of the international symposium and workshop on tropical Peatland, Yogyakarta, EU CARBOPEAT and RESTORPEAT partnership, Gadjah Mada University, Indonesia and University of Leicester, United Kingdom, 27–29 Aug 2007. http://www.geog.le.ac.uk/carbopeat/yogyaproc.html
Van Noordwijk M, Tomich TP, Winahyu R, Murdiyarso D Suyanto, Partoharjono S, Fagi AM (1995) Alternatives to slash-and-burn in Indonesia. Summary report of phase 1. ASB-Indonesia and ICRAF-S.E. Asia. Nairobi, Kenya
Van Noordwijk M, Woomer MP, Cerri C, Bernoux M, Nugroho K (1997) Soil carbon in the humid tropical forest zone. Geoderma 79:187–225. doi:10.1016/S0016-7061(97)00042-6
Wang B, Qiu YL (2006) Phylogenetic distribution and evolution of mycorrhizas in land plants. Mycorrhiza 16:299–363. doi:10.1007/s00572-005-0033-6
White TJ, Bruns T, Lee S, Taylor JW (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR protocols: a guide to methods and applications. Academic Press, Inc., New York, pp 315–322
Whitten J, Damanik SJ, Anwar J, Hisyam N (2000) The ecology of Sumatra. Periplus, Singapore
Williams SE, van Noordwijk M, Penot E, Healey JR, Sinclair FL, Wibawa G (2001) On-farm evaluation of the establishment of clonal rubber in multistrata agroforests in Jambi, Indonesia. Agrofor Syst 53:227–237. doi:10.1023/A:1013336822923
Yasman I (1995) Dipterocarpaceae: tree-mycorrhizae-seedling connections. Ph.D. thesis, Wageningen Agriculture University, The Netherlands
Zhang Z, Schwartz S, Wagner L, Miller W (2000) A greedy algorithm for aligning DNA sequences. J Comput Biol 7:203–214. doi:10.1089/10665270050081478
Acknowledgments
This project was supported by the Netherlands Organization for International Cooperation in Higher Education-Netherlands Fellowship Programme (NUFFIC-NFP) scholarship for H.L.T. and Common Fund for Commodity (CFC) for Smallholder Rubber Agroforestry Systems (SRAS) project-World Agroforestry Centre (ICRAF-SEA). We thank Betha Lusiana and R. Coe for statistical advice; Andree Ekadinata for map design; Douglas Sheil provided critical comments on earlier draft of the manuscript; and two anonymous referees for critical comments. We thank Bert Gerrits van den Ende and Kasper Luijsterburg for laboratory assistance. We would like to thank the Faculty of Forestry, Gadjah Mada University for permission to conduct research in education forest Silvagama, Jambi. Invaluable field assistance and logistical support were provided by Ratna Akiefnawati, Yatni, Najmulah, Sulaeman, Priyatna, Suroto and the participating farmers: Abror, Cholic, Jamhuri, Paijan, Rebiman, Sudirman, Sugiharto, Sunardi, and Supriyadi.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Tata, H.L., van Noordwijk, M., Summerbell, R. et al. Limited response to nursery-stage mycorrhiza inoculation of Shorea seedlings planted in rubber agroforest in Jambi, Indonesia. New Forests 39, 51–74 (2010). https://doi.org/10.1007/s11056-009-9155-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11056-009-9155-6