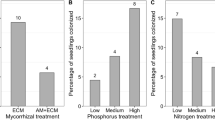
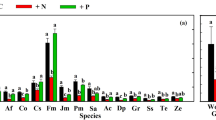
Abstract
We examined the influence of phosphorus source and availability on host plant (Pinus rigida) response to ectomycorrhizal diversity under contrasting P conditions. An ectomycorrhizal richness gradient was established with equimolar P supplied as either inorganic phosphate or organic inositol hexaphosphate. We measured growth and N and P uptake of individual P. rigida seedlings inoculated with one, two, or four species of ectomycorrhizal fungi simultaneously and without mycorrhizas in axenic culture. Whereas colonization of P. rigida by individual species of ectomycorrhizal fungi decreased with increasing fungal richness, colonization of all species combined increased. Plant biomass and N content increased across the ectomycorrhizal richness gradient in the organic but not the inorganic P treatment. Plants grown under organic P conditions had higher N concentration than those grown under inorganic P conditions, but there was no effect of richness. Phosphorus content of plants grown in the organic P treatment increased with increasing ectomycorrhizal richness, but there was no response in the inorganic P treatment. Phosphorus concentration was higher in plants grown at the four-species richness level in the organic P treatment, but there was no effect of diversity under inorganic P conditions. Overall, few ectomycorrhizal composition effects were found on plant growth or nutrient status. Phosphatase activities of individual ectomycorrhizal fungi differed under organic P conditions, but there was no difference in total root system phosphatase expression between the inorganic or organic P treatments or across richness levels. Our results provide evidence that plant response to ectomycorrhizal diversity is dependent on the source and availability of P.





Similar content being viewed by others

References
Abuzinadah RA, Read DJ (1989) The role of proteins in the nitrogen nutrition of ectomycorrhizal plants. V. Nitrogen transfer in birch (Betula pendula) grown in association with mycorrhizal and non-mycorrhizal fungi. New Phytol 112:61–68
Allen SE (1989) Chemical analysis of ecological materials, 2nd edn. Blackwell Scientific Publications, Oxford, UK
Allen EB, Allen MF, Helm DJ, Trappe JM, Molina R, Rincon E (1995) Patterns and regulation of mycorrhizal plant and fungal diversity. Plant Soil 170:47–62
Antibus RK, Sinsabaugh RL, Linkins AE (1992) Phosphatase activities and phosphorus uptake from inositol phosphate by ectomycorrhizal fungi. Can J Bot 70:794–801
Antibus RK, Bower D, Dighton J (1997) Root surface phosphatase activities and uptake of 32P-labeled inositol phosphate in field-collected gray birch and red maple roots. Mycorrhiza 7:39–46
Baxter JW, Dighton J (2001) Ectomycorrhizal diversity alters growth and nutrient acquisition of grey birch (Betula populifolia) seedlings in host–symbiont culture conditions. New Phytol 152:139–149
Bending GD, Read DJ (1995) The structure and function of the vegetative mycelium of ectomycorrhizal plants. V. Foraging behaviour and translocation of nutrients from exploited litter. New Phytol 130:401–409
Bruns TD (1995) Thoughts on the process that maintain local species diversity of ectomycorrhizal fungi. Plant Soil 170:63–73
Colpaert JV, van Laere A, van Tichelen KK, van Assche JA (1997) The use of inositol hexaphosphate as a phosphorus source by mycorrhizal and non-mycorrhizal Scots pine (Pinus sylvestris). Funct Ecol 11:407–415
Conn C, Dighton J (2000) Litter quality influences on decomposition, ectomycorrhizal community structure and mycorrhizal root surface acid phosphatase activity. Soil Biol Biochem 32:489–496
Dighton J (1991) Acquisition of nutrients from organic resources by mycorrhizal autotrophic plants. Experientia 47:362–369
Dighton J, Poskitt JM, Brown TK (1993) Phosphate influx into ectomycorrhizal and saprotrophic fungal hyphae in relation to phosphate supply; a potential method for selection of efficient mycorrhizal species. Mycol Res 97:355–358
Duchesne LC, Ellis BE, Peterson RL (1989) Disease suppression by the ectomycorrhizal fungus Paxillus involutus: contribution of oxalic acid. Can J Bot 67:2726–2730
Farley RA, Fitter AH (1999) Temporal and spatial variation in soil resources in a deciduous woodland. J Ecol 20:67–69
Finlay RD, Ek H, Odham G, Söderström B (1989) Uptake, translocation and assimilation of nitrogen from 15N-labelled ammonium and nitrate sources by intact ectomycorrhizal systems of Fagus sylvatica infected with Paxillus involutus. New Phytol 113:47–55
Gibson F, Deacon JW (1988) Experimental study of establishment of ectomycorrhizas in different regions of birch root systems. Trans Br Mycol Soc 91:239–251
Harrison AF (1987) Soil organic phosphorus: a review of world literature. CAB International, Wallingford, UK
Hooper DU, Vitousek PM (1997) The effects of plant composition and diversity on ecosystem processes. Science 277:1302–1305
Ingestad T, Kähr M (1985) Nutrition and growth of coniferous seedlings at varied relative nitrogen addition rate. Physiol Plant 65:109–116
Jonsson L, Nilsson M-C, Wardle D, Zackrisson O (2001) Context dependent effects of ectomycorrhizal species richness on tree seedling productivity. Oikos 93:353–364
Kroehler C, Antibus RK, Linkins AE (1988) The effects of organic and inorganic phosphorus concentration on the acid phosphatase activity of ectomycorrhizal fungi. Can J Bot 66:750–756
Lawton JH, Brown VK (1994) Redundancy in ecosystems. In: Schulze ED, Mooney HA (eds) Biodiversity and ecosystem functioning. Springer, Berlin Heidelberg New York
Mason PA, Dighton J, Last FT, Wilson J (1983) Procedure for establishing sheathing mycorrhizas on tree seedlings. For Ecol Manag 5:47–53
Molina R, Massicotte H, Trappe JM (1992) Specificity phenomena in mycorrhizal symbiosis: community-ecological consequences and practical implications. In: Allen MF (ed) Mycorrhizal functioning: an integrative plant–fungal process. Chapman and Hall, New York, NY, pp 357–423
Naeem S, Håkansson K, Lawton JH, Crawley MJ, Thompson LJ (1996) Biodiversity and plant productivity in a model assemblage of plant species. Oikos 76:259–264
Palmer JG, Miller OK, Gruhn C (1993) Fruiting of ectomycorrhizal basidiomycetes on unburned and prescribed-burned pine/hardwood plots after drought-breaking rainfalls in the Allegheny Mountains of southwestern Virginia. Mycorrhiza 4:93–104
Parke JL, Linderman RG, Black CH (1983) The role of ectomycorrhizas in drought tolerance of Douglas-fir seedlings. New Phytol 95:83–95
Perez-Moreno J, Read DJ (2000) Mobilization and transfer of nutrients from litter to tree seedlings via the vegetative mycelium of ectomycorrhizal plants. New Phytol 145:301–309
Read DJ (1991) Mycorrhizas in ecosystems. Experientia 47:376–390
Rygiewicz PT, Anderson CP (1994) Mycorrhizae alter quality and quantity of carbon allocated below ground. Nature 369:58–60
SAS Institute Inc. (1990) Statistical analysis software, version 6.01. SAS Institute Inc., Cary, NC
Smith SE, Read DJ (1997) Mycorrhizal symbiosis, 2nd edn. Academic, San Diego, CA
Tennant D (1975) A test of a modified line intersect method of estimating root length. J Ecol 63:995–1001
Tibbett M (2002) Considerations on the use of the p-nitrophenyl phosphomonoesterase assay in the study of the phosphorus nutrition of soil borne fungi. Microbiol Res 157:221–231
Tibbett M, Sanders FE (2002) Ectomycorrhizal symbiosis can enhance plant nutrition through improved access to discrete organic nutrient patches of high resource quality. Ann Bot 89:783–789
Tilman D, Wedin D, Knops J (1996) Productivity and sustainability influenced by biodiversity in grassland ecosystems. Nature 379:718–720
van der Heijden MGA, Klironomos JN, Ursic M, Moutoglis P, Streitwolf-Engel R, Boiler T, Wiemken A, Sanders IR (1998) Mycorrhizal fungal diversity determines plant biodiversity, ecosystem variability and productivity. Nature 396:69–72
Wall DH, Moore JC (1999) Interactions underground: soil biodiversity, mutualism and ecosystem processes. BioScience 49:109–117
Wardle DA (1999) Is “sampling effect” a problem for experiments investigating biodiversity–ecosystem function relationships? Oikos 87:403–407
Acknowledgements
We would like to thank Christine Edly for her diligence in establishing and maintaining the mycorrhizal plants and conducting plant nutrient analyses. Cultures of Pisolithus tinctorius and Piloderma bicolor were generously supplied by O.K. Miller and Cenococcum geophilum was provided by Amy Tuininga. Thanks are also extended to the two anonymous reviewers whose comments significantly improved the manuscript. This research was funded by a National Science Foundation grant IBN-9723254.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baxter, J.W., Dighton, J. Phosphorus source alters host plant response to ectomycorrhizal diversity. Mycorrhiza 15, 513–523 (2005). https://doi.org/10.1007/s00572-005-0359-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00572-005-0359-0