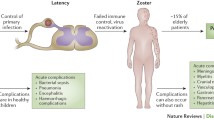
This review presents data on the characteristics of the varicella-zoster virus (VZV), the clinical manifestations of CNS lesions in acute and chronic VZV infection in children and adults, and the mechanisms of interaction of the pathogen with the immune system during development of disease. The question of whether neurological disorders in VZV infection should be regarded as a complication or a manifestation of the disease, caused by a defective virus or the presence of subclinical immunodeficiency – as has been confirmed by contemporary scientific studies – is discussed. The critical mechanisms of immune defense against VZV, the main cause of penetration of the virus into the CNS and the development of neurological disorders, and the relationship between VZV genotypes and the presence of mutations in the gE gene and the nature of the course of illness are described; the review also addresses detection of rare variants of the POLR3A, POLR3C, POLR3E, and POLR3F genes, which are associated with impaired interferon induction and the development of severe VZV infection, in which vasculopathy also occurs and is the basis for the use of complex vascular drugs such as Cytoflavin, whose efficacy has been evidenced by the authors. Particular emphasis is given to analysis of intrathecal immunopathogenesis, which may be associated with the presence and severity of neurological manifestations. The causes of severe disease in patients vaccinated against chicken pox are discussed, along with resistance to specific antiviral drugs, which is probably associated with mutations responsible for therapeutic resistance of the virus.
Similar content being viewed by others
References
Skripchenko, E. Yu., Ivanova, G. P., Lobzin, Yu. V., et al., “Neurological complications in chickenpox: diagnosis and management tactics,” in: Neuroinfections in Children, Skripchenko, N. V. (ed.), Taktik-Studio, St. Petersburg (2015), pp. 349–394.
Weinmann, S., Chun, C., Schmid, D., et al., “Incidence and clinical characteristics of herpes zoster among children in the varicella vaccine era, 2005–2009,” J. Infect. Dis., 208, No. 11, 1859–1868 (2013), https://doi.org/10.1093/infdis/jit405.
Rodríguez-Fanjul, X., Noguera, A., Vicente, A., et al., “Herpes zoster in healthy infants and toddlers after perinatal exposure to varicella-zoster virus: a case series and review of the literature,” Pediatr. Infect. Dis. J., 29, No. 6, 574–576 (2010), https://doi.org/10.1097/INF.0b013e3181d76f7f.
Sabitov, A. U., Fomin, V. V., and Sharova, A. A., “Immunopathogenesis of chickenpox, “Ural. Med. Zh., 6, No. 111, 8–14 (2013).
Zheleznikova, G. F., Skripchenko, N. V., and Skripchenko, E. Y., “Varicella-zoster herpes virus and the immune response,” Ros. Immunol. Zhurn., 7, No. 16, 35–48 (2013).
Lavrov, V. F., Svitich, O. A., Kazanova, A. S., et al., “Varicella zoster virus infection: immunity, diagnosis, and in vivo modeling,” Zh. Mikrobiol. Epidemiol. Immunobiol., 4, 82–89 (2019).
Laing, K., Ouwendijk, W., Koelle, D., et al., “Immunobiology of varicella-zoster virus infection,” J. Infect. Dis., 218, Suppl. 2, S68–S74 (2018), https://doi.org/10.1093/infdis/jiy403.
Sorel, O. and Messaoudi I., “Insights into the pathogenesis of varicella viruses,” Curr. Clin. Microbiol. Rep., 6, No. 3, 156–165 (2019), https://doi.org/10.1007/s40588-019-00119-2.
Grahn, A. and Studahl M., “Varicella-zoster virus infections of the central nervous system – Prognosis, diagnostics and treatment,” J. Infect., 71, No. 3, 281–293 (2015), https://doi.org/10.1016/j.jinf.2015.06.004.
Rack, A., Grote, V., Streng, A., et al., “Neurologic varicella complications before routine immunization in Germany,” Pediatr. Neurol., 42, No. 1, 40–48 (2010), https://doi.org/10.1016/j.pediatrneurol.2009.07.012.
Kazanova, A. S., Lavrov, V. F., and Zverev, V. V., “Varicella-Zoster virus and vascular diseases of the central nervous system,” Zh. Mikrobiol., 3, 106–116 (2015).
Skripchenko, N. V., “Effectiveness of cytoflavin in disseminated encephalomyelitis in children,” Zh. Nevrol. Psikhiatr., 11, No. 2, 67–74 (2017), https://doi.org/10.17116/jnevro201711711267-74.
Heusel, E. and Grose C., “Twelve children with varicella vaccine meningitis: neuropathogenesis of reactivated live attenuated varicella vaccine virus,” Viruses, 12, No. 10, 1078 (2020), https://doi.org/10.3390/v12101078.
Oliver, S., Zhou, M., and Arvin A., “Varicella-zoster virus: molecular controls of cell fusion-dependent pathogenesis,” Biochem. Soc. Trans., 48, No. 6, 2415–2435 (2020), https://doi.org/10.1042/BST20190511.
Nikzad, R., Angelo, L., Aviles-Padilla, K., et al., “Human natural killer cells mediate adaptive immunity to viral antigens,” Sci. Immunol., 4, No. 35, eaat8116 (2019), https://doi.org/10.1126/sciimmunol.aat8116.
Eberhardt, C., Wieland, A., Nasti, T., et al., “Persistence of varicella-zoster virus-specific plasma cells in adult human bone marrow following childhood vaccination,” J. Virol., 94, No. 13, e02127-19 (2020), https://doi.org/10.1128/JVI.02127-19.
Sen, N., Sung, P., Panda, A., and Arvin A., “Distinctive roles for type I and type II interferons and interferon regulatory factors in the host cell defense against varicella-zoster virus,” J. Virol., 92, No. 21, e01151-18 (2018), https://doi.org/10.1128/JVI.01151-18.
Horien, C. and Grose C., “Neurovirulence of varicella and the live attenuated varicella vaccine virus,” Semin. Ped. Neurol., 19, No. 3, 124–129 (2012), https://doi.org/10.1016/j.spen.2012.02.006.
Vossen, M., Biezeveld, M., de Jong, M., et al., “Absence of circulating natural killer and primed CD8+ cells in life-threatening varicella,” J. Infect. Dis., 191, No. 2, 198–206 (2005), https://doi.org/10.1086/426866.
Karadag, O., Kara, A., Celik, M., et al., “Determination and clinical correlation of markers of inflammation in unvaccinated patients with varicella-zoster infection,” Eur. Rev. Med. Pharmacol. Sci., 17, No. 15, 2032–2039 (2013).
Saburova, O. A., Butina T. Yu, Ryumin, A. M., et al., “Immunological criteria for prognosticating severe and complicated forms of chickenpox,” Sovrem. Tekhnol. Med., 12, No. 4, 48–54 (2020), https://doi.org/10.17691/stm2020.12.4.06.
Kennedy, P. and Mogensen T., “Determinants of neurological syndromes caused by varicella zoster virus (VZV),” J. Neurovirol., 26, No. 4, 482–495 (2020), https://doi.org/10.1007/s13365-020-00857-w.
Sharova, A. A. and Sabitov, A. U., “Clinical and immunological features of severe CP with CNS lesions in children,” Sist. Integr. Zdravookhr., 11, No. 1, 23–33 (2011).
Zheleznikova, G. F., Lobzin, Yu. V., and Skripchenko, N. V., et al., “Clinical significance of serum cytokine levels in chickenpox in children,” Infekts. Immun., 5, No. 1, 79–84 (2015), https://doi.org/10.15789/2220-7619-2015-1-79-84.
Bozzola, E., Carsetti, R., Mortari, E., et al., “The link between varicella and immune system: which children will develop acute cerebellitis?” Ital. J. Pediatr., 46, No. 1, 75–79 (2020), https://doi.org/10.1186/s13052-020-00840-5.
Steain, M., Sutherland, J., Rodriguez, M., et al., “Analysis of T cell responses during active varicella-zoster virus reactivation in human ganglia,” J. Virol., 88, No. 5, 2704–2716 (2014), https://doi.org/10.1128/JVI.03445-13.
Schub, D., Janssen, E., Leyking, S., et al., “Altered phenotype and functionality of varicella zoster virus-specific cellular immunity in individuals with active infection,” J. Infect. Dis., 211, No. 4, 600–612 (2015), https://doi.org/10.1093/infdis/jiu500.
Schub, D., Fousse, M., Faßbender, K., et al., “CTLA-4-expression on VZV-specific T cells in CSF and blood is specifically increased in patients with VZV related central nervous system infections,” Eur. J. Immunol., 48, No. 1, 151–160 (2018), https://doi.org/10.1002/eji.201747079.
Lind, L., Eriksson, K., and Grahn A., “Chemokines and matrix metalloproteinases in cerebrospinal fluid of patients with central nervous system complications caused by varicella-zoster virus,” J. Neuroinflammation, 16, No. 1, 42 (2019), https://doi.org/10.1186/s12974-019-1428-1.
Casanova, J. L., “Severe infectious diseases of childhood as monogenic inborn errors of immunity,” Proc. Natl. Acad. Sci. USA, 112, No. 51, E7128-37 (2015), https://doi.org/10.1073/pnas.1521651112.
Ogunjimi, B., Zhang, S. Y., Sørensen, K., et al., “Inborn errors in RNA polymerase III underlie severe varicella zoster virus infections,” J. Clin. Invest., 127, No. 9, 3543–3556 (2017), https://doi.org/10.1172/JCI92280.
Carter-Timofte, M., Hansen, A., Christiansen, M., et al., “Mutations in RNA polymerase III genes and defective DNA sensing in adults with varicella-zoster virus CNS infection,” Genes Immun., 20, No. 3, 214–223 (2019), https://doi.org/10.1038/s41435-018-0027-y.
Rottenstreich, A., Oz, Z., and Oren I., “Association between viral load of varicella zoster virus in cerebrospinal fluid and the clinical course of central nervous system infection,” Diagn. Microbiol. Infect. Dis., 79, No. 2, 174–177 (2014), https://doi.org/10.1016/j.diagmicrobio.2014.02.015.
Grahn, A., Bergström, T., Runesson, J., and Studahl M., “Varicellazoster virus (VZV) DNA in serum of patients with VZV central nervous system infections,” J. Infect., 73, No. 3, 254–260 (2016), https://doi.org/10.1016/j.jinf.2016.04.035.
Campbell, T., McSharry, B., Steain, M., et al., “Functional paralysis of human natural killer cells by alphaherpesviruses,” PLoS Pathog., 15, No. 6, e1007784 (2019), https://doi.org/10.1371/journal.ppat.1007784.
Gerada, C., Campbell, T., Kennedy, J., et al., “Manipulation of the innate immune response by varicella zoster virus,” Front. Immunol., 11, 1–17 (2020), https://doi.org/10.3389/fimmu.2020.00001.
Gerada, C., Steain, M., Campbell, T., et al., “Granzyme B cleaves multiple herpes simplex virus 1 and varicella-zoster virus (VZV) gene products, and VZV ORF4 inhibits natural killer cell cytotoxicity,” J. Virol., 93, No. 22, e01140-19 (2019), https://doi.org/10.1128/JVI.01140-19.
Zerboni, L., Sen, N., Oliver, S., and Arvin A., “Molecular mechanisms of varicella zoster virus pathogenesis,” Nat. Rev. Microbiol., 12, No. 3, 197–210 (2014), https://doi.org/10.1038/nrmicro3215.
Mahalingam, R., Gershon, A., Gershon, M., et al., “Current in vivo models of varicella-zoster virus neurotropism,” Viruses, 11, No. 6, 502 (2019), https://doi.org/10.3390/v11060502.
Suenaga, T., Matsumoto, M., Arisawa, F., et al., “Sialic acids on varicella-zoster virus glycoprotein B are required for cell-cell fusion,” J. Biol. Chem., 290, No. 32, 19833–19843 (2015), https://doi.org/10.1074/jbc.M114.635508.
Pugazhenthi, S., Nair, S., Velmurugan, K., et al., “Varicella-zoster virus infection of differentiated human neural stem cells,” J. Virol., 85, No. 13, 6678–6686 (2011), https://doi.org/10.1128/JVI.00445-11.
Selariu, A., Cheng, T., Tang, Q., et al., “ORF7 of varicella-zoster virus is a neurotropic factor,” J. Virol., 86, No. 16, 8614–8624 (2012), https://doi.org/10.1128/JVI.00128-12.
Santos, R., Padilla, J., Hatfield, C., and Grose C., “Antigenic variation of varicella zoster virus Fc receptor gE: loss of a major B cell epitope in the ectodomain,” Virology, 249, No. 1, 21–31 (1998), https://doi.org/10.1006/viro.1998.9313.
Santos, R., Hatfield, C., Cole, N., et al., “Varicella-zoster virus gE escape mutant VZV-MSP exhibits an accelerated cell-to-cell spread phenotype in both infected cell cultures and SCID-hu mice,” Virology, 275, No. 2, 306–317 (2000), https://doi.org/10.1006/viro.2000.0507.
Grose, C., Tyler, S., Peters, G., et al., “Complete DNA sequence analyses of the first two varicella-zoster virus glycoprotein E (D150N) mutant viruses found in North America: evolution of genotypes with an accelerated cell spread phenotype,” J. Virol., 78, No. 13, 6799–6807 (2004), https://doi.org/10.1128/JVI.78.13.6799-6807.2004.
Natoli, S., Ciotti, M., Paba, P., et al., “A novel mutation of varicella-zoster virus associated to fatal hepatitis,” J. Clin. Virol., 37, No. 1, 72–74 (2006), https://doi.org/10.1016/j.jcv.2006.06.004.
Schmidt-Chanasit, J., Bleymehl, K., Schäd, S., et al., “Novel varicella-zoster virus glycoprotein E gene mutations associated with genotypes A and D,” J. Clin. Microbiol., 46, No. 1, 325–327 (2008), https://doi.org/10.1128/JCM.01735-07.
Loparev, V., Rubtcova, E., Bostik, V., et al., “Distribution of varicella-zoster virus (VZV) wild-type genotypes in northern and southern Europe: evidence for high conservation of circulating genotypes,” Virology, 383, No. 2, 216–225 (2009), https://doi.org/10.1016/j.virol.2008.10.026.
Sauerbrei, A., Bohn, K., Zell, R., and Wutzler P., “Variability of immediate-early gene 62 in German varicella-zoster virus wild-type strains,” J. Clin. Microbiol., 47, No. 11, 3717–3720 (2009), https://doi.org/10.1128/JCM.01550-09.
Breuer, J., Grose, C., Norberg, P., et al., “A proposal for a common nomenclature for viral clades that form the species varicella-zoster virus: summary of VZV Nomenclature Meeting 2008, Barts and the London School of Medicine and Dentistry, 24–25 July 2008,” J. Gen. Virol., 91, Pt. 4, 821–828 (2010), https://doi.org/10.1099/vir.0.017814-0.
Zell, R., Taudien, S., Pfaff, F., et al., “Sequencing of 21 varicella-zoster virus genomes reveals two novel genotypes and evidence of recombination,” J. Virol., 86, No. 3, 1608–1622 (2012), https://doi.org/10.1128/JVI.06233-11.
Norberg, P., Depledge, D., Kundu, S., et al., “Recombination of globally circulating varicella-zoster virus,” J. Virol., 89, No. 14, 7133–7146 (2015), https://doi.org/10.1128/JVI.00437-15.
Jensen, N., Rivailler, P., Tseng, H., et al., “Revisiting the genotyping scheme for varicella-zoster viruses based on whole-genome comparisons,” J. Gen. Virol., 98, No. 6, 1434–1438 (2017), https://doi.org/10.1099/jgv.0.000772.
Sauerbrei, A., Wiesener, N., Zell, R., and Wutzler P., “Sequence analysis of the glycoprotein E gene of varicella-zoster virus strains of clades 1, 3 and 5,” Arch. Virol., 156, No. 3, 505–509 (2011), https://doi.org/10.1007/s00705-010-0864-0.
Breuer J., “Molecular genetic insights into varicella zoster virus (VZV), the vOka vaccine strain, and the pathogenesis of latency and reactivation,” J. Infect. Dis., 218, No. 2, S75–S80 (2018), https://doi.org/10.1093/infdis/jiy279.
Pontremoli, C., Forni, D., Clerici, M., et al., “Possible European origin of circulating varicella zoster virus strains,” J. Infect. Dis., 221, No. 8, 1286–1294 (2020), https://doi.org/10.1093/infdis/jiz227.
Beby-Defaux, A., Brabant, S., Chatellier, D., et al., “Disseminated varicella with multiorgan failure in an immunocompetent adult,” J. Med. Virol., 81, No. 4, 747–749 (2009), https://doi.org/10.1002/jmv.21447.
Springfeld, C., Sauerbrei, A., Filusch, A., et al., “Fatal varicella in an immunocompromised adult associated with a European genotype E2 variant of varicella zoster virus,” J. Clin. Virol., 44, No. 1, 70–73 (2009), https://doi.org/10.1016/j.jcv.2008.10.004.
Plisek, S., Pliskova, L., Bostik, V., et al., “Fulminant hepatitis and death associated with disseminated varicella in an immunocompromised adult from the Czech Republic caused by a wild-type clade 4 varicella-zoster virus strain,” J. Clin. Virol., 50, No. 1, 72–75 (2011), https://doi.org/10.1016/j.jcv.2010.09.014.
Piret, J. and Boivin G., “Antiviral resistance in herpes simplex virus and varicella-zoster virus infections: diagnosis and management,” Curr. Opin. Infect. Dis., 29, No. 6, 654–662 (2016), https://doi.org/10.1097/QCO.0000000000000288.
Gueudry, J., Boutolleau, D., Gueudin, M., et al., “Acyclovir-resistant varicella-zoster virus keratitis in an immunocompetent patient,” J. Clin. Virol., 58, No. 1, 318–320 (2013), https://doi.org/10.1016/j.jcv.2013.04.024.
van der Beek, M., Vermont, C., Bredius, R., et al., “Persistence and antiviral resistance of varicella zoster virus in hematological patients,” Clin. Infect. Dis., 56, No. 3, 335–343 (2013), https://doi.org/10.1093/cid/cis879.
Hoffmann, A., Döring, K., Seeger, N., et al., “Genetic polymorphism of thymidine kinase (TK) and DNA polymerase (pol) of clinical varicella-zoster virus (VZV) isolates collected over three decades,” J. Clin. Virol., 95, 61–65 (2017), https://doi.org/10.1016/j.jcv.2017.08.011.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Zhurnal Nevrologii i Psikhiatrii imeni S. S. Korsakova, Vol. 122, No. 10, pp. 46–56, October, 2022.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Skripchenko, E.Y., Zheleznikova, G.F., Skripchenko, N.V. et al. Immunopatological and Genetic Aspects of the Pathogenesisof CNS Lesions in VZV Infection. Neurosci Behav Physi 53, 801–811 (2023). https://doi.org/10.1007/s11055-023-01472-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11055-023-01472-y